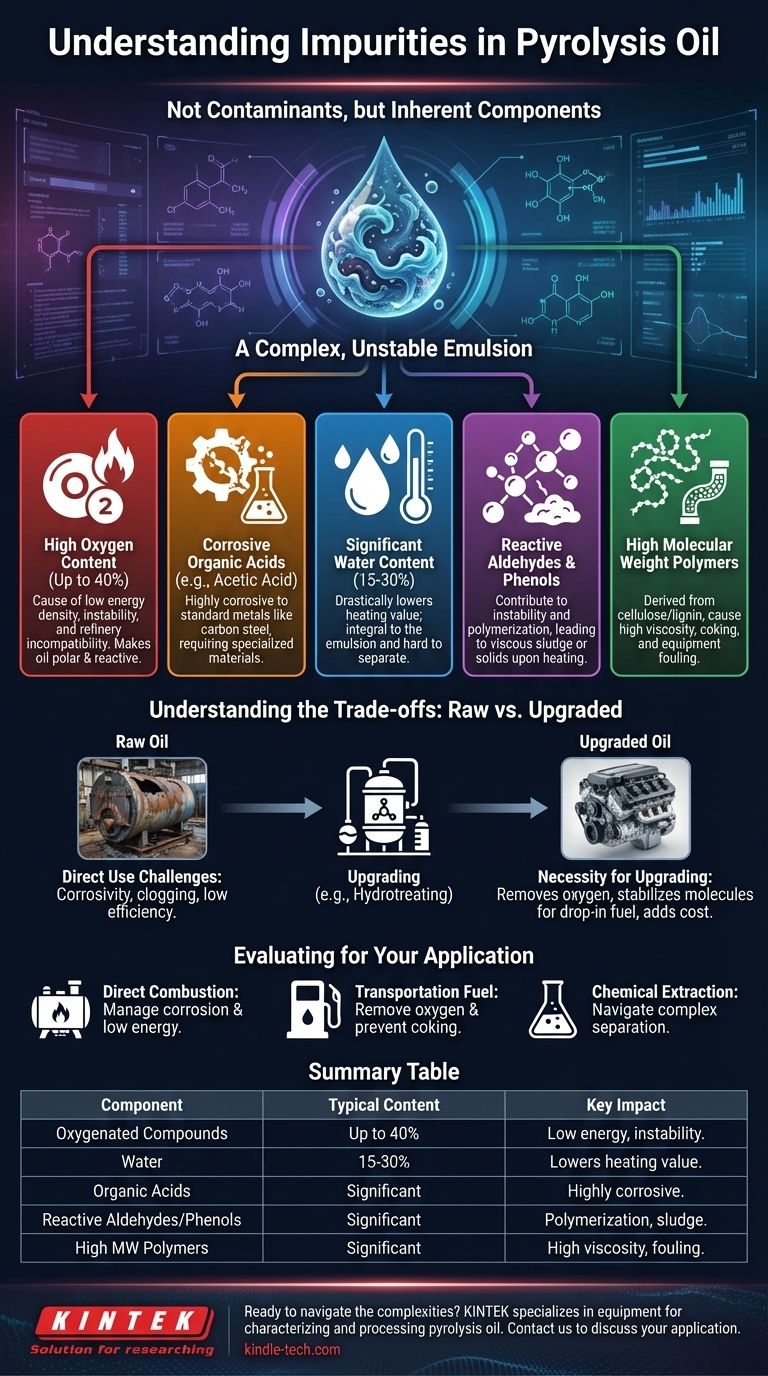
The primary impurities in pyrolysis oil are not contaminants in the traditional sense, but rather inherent components of its complex chemical structure. These include a high concentration of oxygenated organic compounds, significant water content, and various reactive polymers, which make the oil unstable and corrosive.
The core challenge of pyrolysis oil is that its "impurities" are its very nature. Its high oxygen and water content, along with reactive acids and polymers, make it fundamentally different from conventional crude oil, requiring significant upgrading before it can be widely used.

The Fundamental Challenge: A Complex Emulsion
Not a True Oil
Pyrolysis oil, also known as bio-crude, is not a hydrocarbon like crude oil. It is a micro-emulsion containing hundreds of different organic compounds chemically dissolved in water.
Inherently Unstable
This complex mixture is thermodynamically unstable. The components can react with each other over time or when heated, causing the oil to thicken, separate, and form solid char.
Key Components and Their Impact
High Oxygen Content
The most significant "impurity" is oxygen, which can be up to 40% of the oil's weight. Oxygen is present in nearly every molecule, making the oil polar and reactive.
This high oxygen content is the root cause of the oil's low energy density, chemical instability, and incompatibility with conventional refinery equipment.
Corrosive Organic Acids
Pyrolysis oil contains low molecular weight compounds like acetic acid and formic acid. These acids make the oil highly corrosive to standard metals like carbon steel, requiring specialized and more expensive materials for storage and transport.
Water Content
Water is a major component, often making up 15-30% of the oil's volume. It is not easily separated as it is integral to the emulsion.
This high water content drastically lowers the heating value of the oil, meaning more fuel is required to produce the same amount of energy.
Reactive Aldehydes and Phenols
Compounds like formaldehyde and various phenols are highly reactive. They contribute to the oil's instability, leading to polymerization—a process where small molecules combine to form large, viscous sludge or solids, especially when heated.
High Molecular Weight Polymers
The oil also contains heavy, complex molecules derived from the breakdown of cellulose and lignin, such as oligosaccharides. These contribute to high viscosity, making the oil difficult to pump, and can easily coke or form char, fouling equipment.
Understanding the Trade-offs: Raw vs. Upgraded Oil
The Problem with Direct Use
Using raw pyrolysis oil directly is challenging. Its corrosivity damages standard boilers and engines, its instability can clog fuel lines, and its low energy density makes it inefficient for many applications.
The Necessity of Upgrading
To be used as a drop-in fuel or refinery feedstock, pyrolysis oil must be "upgraded." This involves chemical processes, like hydrotreating, that use catalysts and hydrogen to remove oxygen and stabilize the reactive molecules. This adds significant cost and complexity.
Evaluating Pyrolysis Oil for Your Application
Understanding these inherent properties is the first step in determining the viability of pyrolysis oil for a specific purpose.
- If your primary focus is direct combustion in a modified boiler: Your main concerns will be managing the corrosive acids and the low energy density caused by the high water content.
- If your primary focus is upgrading to a transportation fuel: Your main challenge is removing the high oxygen content and stabilizing the reactive phenols and polymers to prevent coking during processing.
- If your primary focus is extracting valuable chemicals: Your goal is to navigate the incredibly complex mixture to separate target compounds like phenols from the acids, water, and sugars.
Ultimately, recognizing that pyrolysis oil's "impurities" are its fundamental chemistry is the key to unlocking its potential as a renewable resource.
Summary Table:
| Impurity/Component | Typical Content | Key Impact |
|---|---|---|
| Oxygenated Compounds | Up to 40% wt. | Low energy density, chemical instability, incompatibility with refineries |
| Water | 15-30% vol. | Lowers heating value, integral to emulsion |
| Organic Acids (e.g., Acetic Acid) | Significant | Highly corrosive to standard metals |
| Reactive Aldehydes & Phenols | Significant | Causes polymerization, leading to sludge and solids |
| High MW Polymers (e.g., Oligosaccharides) | Significant | High viscosity, coking, and equipment fouling |
Ready to navigate the complexities of pyrolysis oil analysis or upgrading?
KINTEK specializes in providing high-quality lab equipment and consumables essential for characterizing and processing pyrolysis oil. Whether you need analytical instruments to measure oxygen content, corrosion-resistant materials for handling acids, or reactors for upgrading studies, our solutions are designed to meet the rigorous demands of bioenergy and chemical research.
Contact us today (#ContactForm) to discuss how our expertise can support your specific application, from direct combustion to fuel upgrading and chemical extraction.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- Assemble Lab Cylindrical Press Mold
People Also Ask
- What are the benefits of RF sputtering? Versatile, Stable Thin-Film Deposition
- What is the feedstock for bio-oil production? Choosing the Right Biomass for Optimal Yield and Quality
- At what temperature does THC distillate degrade? A Guide to Preserving Potency and Purity
- How is additive manufacturing used in industry? Unlock Complex, Lightweight, and Custom Parts
- What is sputtering in simple terms? A Guide to High-Quality Thin Film Deposition
- What critical reaction conditions does a shaking incubator provide? Optimize Cassava Cellulose Enzymatic Hydrolysis
- What are the functions of a laboratory stirring system in enhancing the leaching efficiency of gold scrap?
- What are the applications of stainless steel? Unlock Its Versatility for Your Project



















