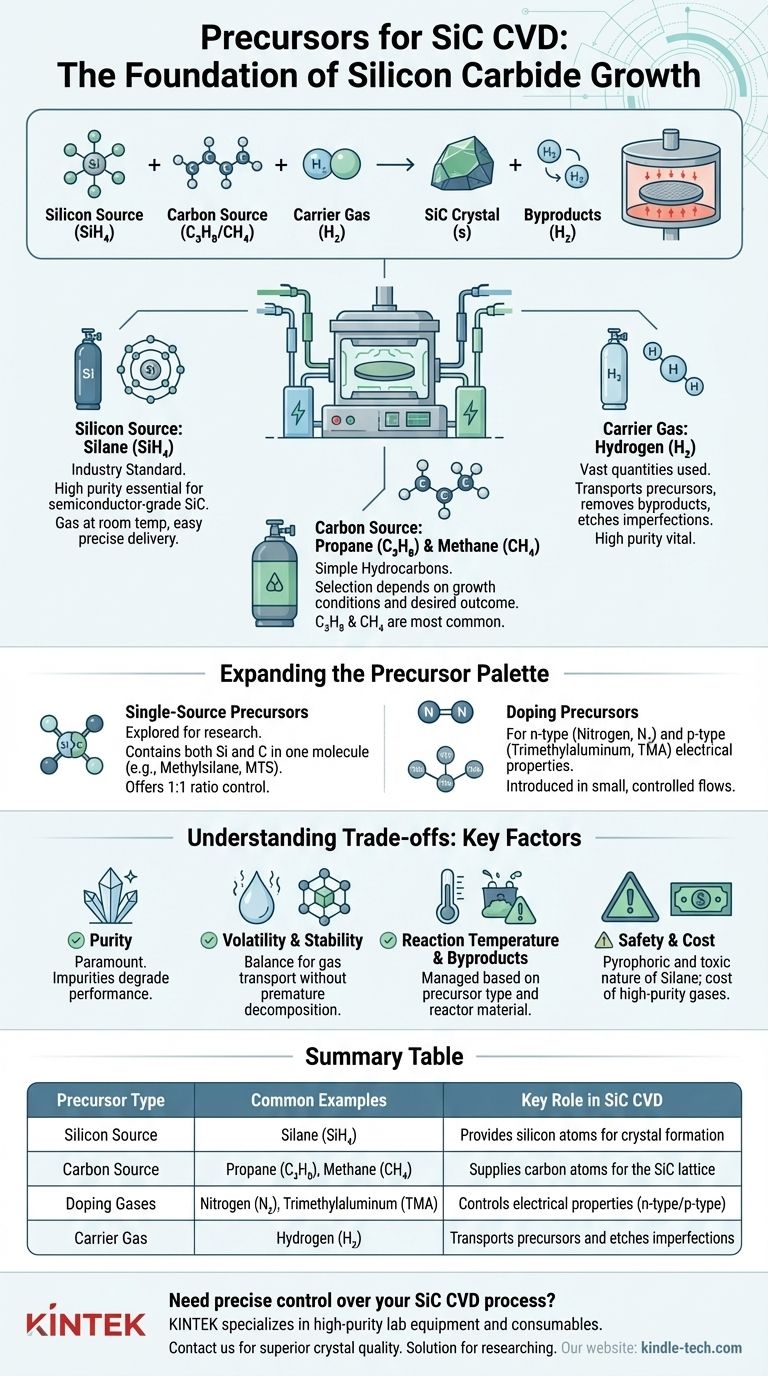
In Silicon Carbide (SiC) Chemical Vapor Deposition (CVD), the most common precursors are a combination of a silicon source gas and a carbon source gas. Typically, silane (SiH4) is used for the silicon, and a simple hydrocarbon like propane (C3H8) or methane (CH4) is used for the carbon, all transported by a carrier gas like hydrogen (H2).
The core principle of SiC CVD is not just about finding any source of silicon and carbon. It is about selecting highly pure, stable, and volatile precursor gases that can be precisely controlled to react at high temperatures, forming a perfect crystalline SiC layer on a substrate.

The Foundation: How SiC CVD Works
The creation of high-quality SiC crystals is a process of atomic-level engineering. The choice of precursor chemicals is the first and most critical step in defining the properties of the final material.
The Core Reaction
At its heart, the process involves the thermal decomposition of the precursor gases on a heated substrate, typically a silicon or SiC wafer. The silicon and carbon atoms then arrange themselves into the desired SiC crystal lattice. The simplified reaction using silane and propane is:
3 SiH4 (g) + C3H8 (g) → 3 SiC (s) + 10 H2 (g)
This reaction occurs at very high temperatures, often exceeding 1500°C, inside the CVD reactor.
Silicon Source: Silane (SiH4)
Silane (SiH4) is the industry standard for the silicon source in SiC epitaxy. It is a gas at room temperature, making it relatively easy to handle and deliver into the reactor with high precision using mass flow controllers. Its high purity is essential for producing semiconductor-grade material.
Carbon Source: Propane (C3H8) vs. Methane (CH4)
The carbon source is typically a simple hydrocarbon. Propane (C3H8) and methane (CH4) are the two most common choices. The selection between them often depends on the specific growth conditions and desired outcome, as their decomposition temperatures and reaction kinetics differ.
The Carrier Gas: Hydrogen (H2)
Vast quantities of purified hydrogen (H2) are used as a carrier gas. It serves two purposes: it transports the precursor gases into the reactor, and it helps to remove unwanted byproducts and etch away imperfections from the growing crystal surface, improving overall quality.
Expanding the Precursor Palette
While the silane-propane system is the workhorse for high-quality SiC growth, other precursors are used for specific applications, including doping and research into alternative growth methods.
Single-Source Precursors
To simplify the process, researchers have explored single-source precursors that contain both silicon and carbon in one molecule. Examples include methylsilane (CH3SiH3) or methyltrichlorosilane (CH3SiCl3). The idea is to have a 1:1 ratio of Si to C atoms built into the molecule, potentially offering better control, though these are less common in mass production.
Precursors for Doping
To be useful in electronics, SiC must be doped to become n-type or p-type. This is achieved by introducing a small, controlled flow of a third precursor during growth.
- N-type doping (adding electrons) is almost always done using Nitrogen (N2) gas.
- P-type doping (adding "holes") is commonly achieved with Trimethylaluminum (TMA).
Understanding the Trade-offs
Choosing a precursor system involves balancing several critical factors. There is no single "best" set of precursors, only the right set for a specific goal.
Purity is Paramount
The electronic properties of SiC are extremely sensitive to impurities. Any contaminants in the precursor gases can become incorporated into the crystal lattice, acting as defects that degrade device performance. This is why semiconductor-grade (e.g., 99.9999% pure) gases are required.
Volatility and Stability
A precursor must be volatile enough to be transported as a gas but stable enough not to decompose before it reaches the hot wafer surface. Premature decomposition can lead to powder formation in the reactor, ruining the crystal growth.
Reaction Temperature and Byproducts
Different precursors react at different temperatures and produce different chemical byproducts. A process that uses chlorinated precursors, for example, must be managed in a reactor resistant to corrosion from hydrochloric acid (HCl) byproducts.
Safety and Cost
Precursors like silane are pyrophoric (ignite spontaneously in air) and toxic, requiring extensive safety infrastructure. The cost and availability of ultra-high-purity gases are also significant factors in a production environment.
Making the Right Choice for Your Goal
Your selection of a precursor system is determined entirely by the intended application of the SiC material.
- If your primary focus is high-quality power electronic devices: Stick to the industry-standard high-purity silane (SiH4) and propane (C3H8) system, with nitrogen (N2) and TMA for controlled doping.
- If your primary focus is research into lower-temperature growth: Exploring single-source precursors or alternative carbon sources might yield novel results.
- If your primary focus is cost-effective bulk crystal growth: Processes using precursors like methyltrichlorosilane (MTS) have historically been used and may be relevant.
Mastering SiC growth is ultimately about controlling the precise chemistry delivered by these foundational precursor molecules.
Summary Table:
| Precursor Type | Common Examples | Key Role in SiC CVD |
|---|---|---|
| Silicon Source | Silane (SiH₄) | Provides silicon atoms for crystal formation |
| Carbon Source | Propane (C₃H₈), Methane (CH₄) | Supplies carbon atoms for the SiC lattice |
| Doping Gases | Nitrogen (N₂), Trimethylaluminum (TMA) | Controls electrical properties (n-type or p-type) |
| Carrier Gas | Hydrogen (H₂) | Transports precursors and etches imperfections |
Need precise control over your SiC CVD process? KINTEK specializes in high-purity lab equipment and consumables, including gas delivery systems and reactors designed for semiconductor-grade SiC growth. Our solutions ensure the stability, purity, and safety required for superior crystal quality. Contact us today to optimize your CVD process and achieve breakthrough results!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- CVD Diamond Domes for Industrial and Scientific Applications
- CVD Diamond Cutting Tool Blanks for Precision Machining
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- What are the advantages of industrial CVD for solid boriding? Superior Process Control and Material Integrity
- How does chirality affect carbon nanotubes? It Determines If They Are Metal or Semiconductor
- What function does CVD equipment serve in rhodium-modified coatings? Achieve Deep Diffusion and Microstructural Precision
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques
- Why are carbon nanotubes important in industry? Unlocking Next-Generation Material Performance



















