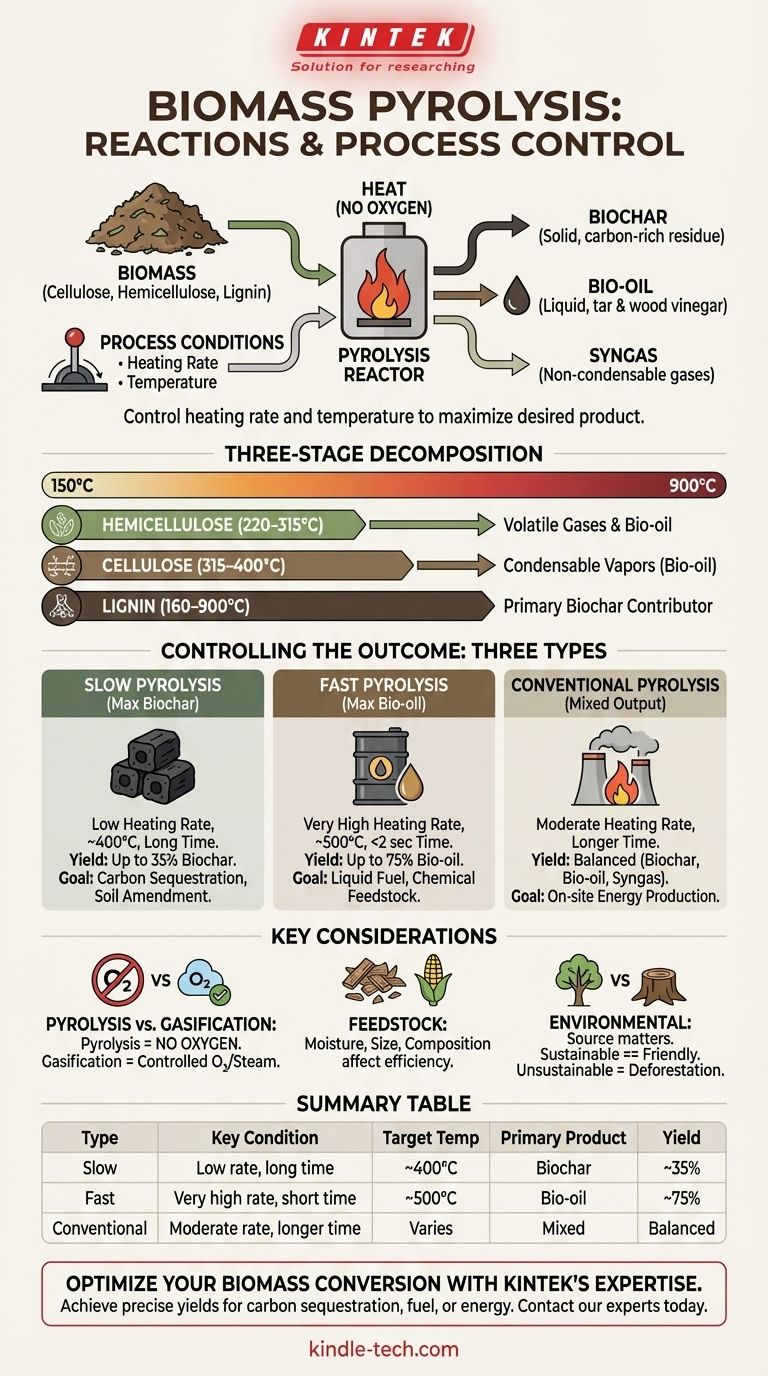
To be precise, biomass pyrolysis isn't a single chemical reaction but a complex series of reactions where heat decomposes organic material in the absence of oxygen. The process breaks down the primary components of biomass—cellulose, hemicellulose, and lignin—into three main products: a solid carbon-rich residue called biochar, a liquid known as bio-oil (which includes tar and wood vinegar), and a mixture of non-condensable gases called syngas.
The central principle of pyrolysis is that you can control the final product mix by manipulating the process conditions. How fast you apply heat and the final temperature you reach are the primary levers that determine whether you maximize the output of solid biochar, liquid bio-oil, or combustible syngas.

The Core Mechanism: A Three-Stage Breakdown
Biomass is primarily composed of three complex polymers. Pyrolysis works by thermally cracking these large molecules into smaller, more useful ones. Each component decomposes at a different temperature range, influencing the overall process.
Stage 1: Hemicellulose Decomposition
Hemicellulose is the first component to break down, typically in the temperature range of 220–315°C. Its decomposition yields a mix of volatile gases and some bio-oil and char.
Stage 2: Cellulose Decomposition
Cellulose, a more stable polymer, decomposes in a narrower and higher temperature range, around 315–400°C. This stage is crucial for producing the condensable vapors that form bio-oil.
Stage 3: Lignin Decomposition
Lignin is the most resilient component and breaks down slowly across a very wide temperature range, from 160–900°C. It is the primary contributor to the final biochar yield due to its complex, aromatic structure that is difficult to break apart.
Controlling the Outcome: The Three Types of Pyrolysis
The specific operating conditions of a pyrolysis reactor dictate the proportion of solid, liquid, and gas products. Engineers have developed three main approaches to target different outcomes.
Slow Pyrolysis for Biochar
This method uses low heating rates and relatively low temperatures (around 400°C) with long residence times. These conditions are optimized to maximize the production of the solid residue, yielding up to 35% biochar. This is the traditional method used for making charcoal.
Fast Pyrolysis for Bio-oil
Fast (or rapid) pyrolysis uses very high heating rates and moderate temperatures (around 500°C) with an extremely short residence time (often less than 2 seconds). These conditions are designed to quickly vaporize the biomass and then rapidly cool the vapors to maximize the yield of liquid bio-oil, which can be as high as 75%.
Conventional Pyrolysis for a Mixed Output
Also known as intermediate pyrolysis, this is a balanced approach. It uses lower heating rates and longer residence times than fast pyrolysis. The result is a more evenly distributed output of biochar, bio-oil, and syngas, without maximizing any single product.
Key Considerations and Common Misconceptions
Understanding the boundaries of the process is critical for its successful application. Pyrolysis has specific requirements and is often confused with similar thermochemical processes.
Misconception: Pyrolysis vs. Gasification
Pyrolysis is defined by the absence of an oxidizing agent like oxygen. Gasification, a related process, intentionally introduces a small, controlled amount of oxygen, steam, or air. This allows for reactions like C + O2 = CO2 and C + H2O = CO + H2, which are characteristic of gasification, not pure pyrolysis.
The Role of Feedstock
The type and quality of biomass used (e.g., wood chips, agricultural waste) significantly impact the process. The moisture content, particle size, and chemical composition of the feedstock must be carefully managed for efficient and predictable results.
Environmental Trade-offs
While pyrolysis can create environmentally friendly products like carbon-sequestering biochar and renewable bio-oil, the overall impact depends on the source of the biomass. Using unsustainable sources can lead to deforestation and habitat loss, negating the potential benefits.
Making the Right Choice for Your Goal
The optimal pyrolysis strategy depends entirely on the desired end product. Your goal determines the necessary process conditions.
- If your primary focus is carbon sequestration or soil amendment: Slow pyrolysis is the ideal path, as it is specifically designed to maximize the yield of stable, solid biochar.
- If your primary focus is producing a liquid fuel or chemical feedstock: Fast pyrolysis is the correct choice to maximize the conversion of biomass into liquid bio-oil.
- If your primary focus is on-site energy production with multiple outputs: Conventional pyrolysis provides a balanced mix of solid, liquid, and gaseous fuels that can be used for heat and power.
Ultimately, mastering pyrolysis is about understanding how to precisely control heat and time to deconstruct biomass into its most valuable forms.
Summary Table:
| Pyrolysis Type | Key Condition | Target Temperature | Primary Product | Yield |
|---|---|---|---|---|
| Slow Pyrolysis | Low heating rate, long residence time | ~400°C | Biochar | Up to 35% |
| Fast Pyrolysis | Very high heating rate, short residence time (<2 sec) | ~500°C | Bio-oil | Up to 75% |
| Conventional Pyrolysis | Moderate heating rate, longer residence time | Varies | Mixed (Biochar, Bio-oil, Syngas) | Balanced |
Ready to optimize your biomass conversion process? Whether your goal is to maximize biochar for carbon sequestration, produce bio-oil for fuel, or generate syngas for energy, KINTEK's expertise in laboratory pyrolysis equipment is your key to success. Our specialized reactors and consumables are designed for precise temperature control and process optimization, ensuring you achieve the exact product yields you need. Contact our experts today to discuss how we can support your specific biomass pyrolysis research and development goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Three-dimensional electromagnetic sieving instrument
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















