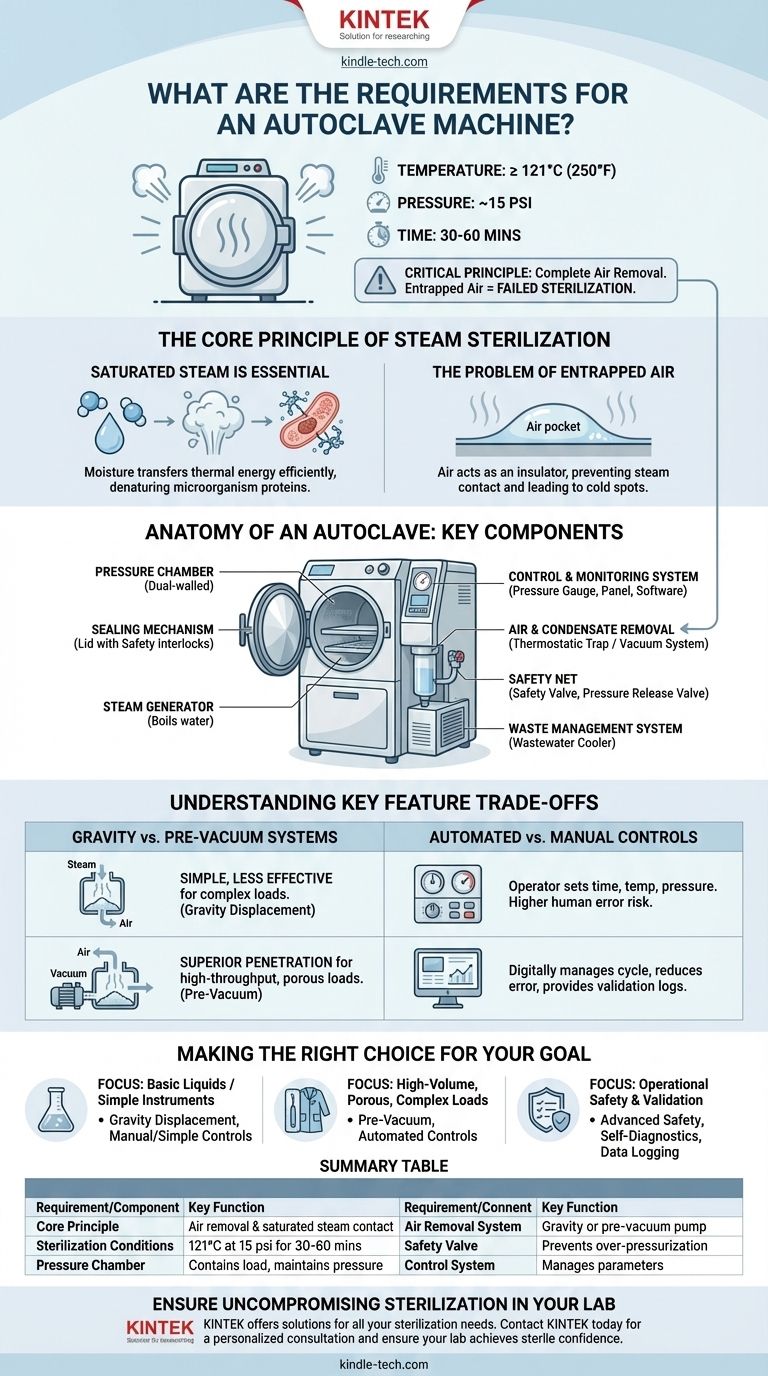
At its core, an autoclave requires the ability to generate and contain saturated steam under high pressure. The fundamental operational requirement is to achieve a chamber temperature of at least 250°F (121°C) at a pressure of approximately 15 pounds per square inch (psi) and hold these conditions for a prescribed time, typically 30 to 60 minutes. This environment is what ensures the complete sterilization of its contents.
The effectiveness of an autoclave isn't just about heat and pressure; it hinges on a critical principle: the complete removal of air from the chamber. If air is present, it prevents the saturated steam from making direct contact with all surfaces, which compromises and ultimately prevents true sterilization.

The Core Principle of Steam Sterilization
Why Saturated Steam is Essential
An autoclave works by subjecting items to high-pressure saturated steam. This process is far more effective than using dry heat.
The moisture in the steam efficiently transfers thermal energy to microorganisms, causing their essential proteins to coagulate and denature, which is lethal.
The Problem of Entrapped Air
Air is an insulator and acts as a barrier, preventing steam from reaching every surface of the instruments inside the chamber.
If air pockets are trapped within the load, those areas will not reach the required sterilization temperature, leading to a failed cycle. Therefore, every functional autoclave must have a mechanism to remove air before or during the sterilization process.
Anatomy of an Autoclave: Key Components
An autoclave is a system of components working in concert to create and maintain a highly controlled environment.
The Pressure Chamber
This is the main vessel where sterilization occurs. It is typically a dual-walled structure with an inner chamber to hold the items and an outer jacket that steam can circulate through to help maintain a uniform temperature.
The Sealing Mechanism (Lid or Door)
The lid creates an airtight seal, which is absolutely essential for building and maintaining the high pressure required for sterilization. Modern autoclaves have safety interlocks that prevent the door from being opened while the chamber is pressurized.
The Steam Generator
This component, often an integrated electric heater, boils water to create the necessary saturated steam. In some large facilities, steam may be supplied from a central boiler instead of an internal generator.
The Control and Monitoring System
This includes the pressure gauge to display the internal pressure and the control panel or software that manages the sterilization cycle. Modern controls allow for adjustable temperature and time settings and often include self-diagnostic programs.
Air and Condensate Removal
A thermostatic trap or vent allows cooler air and condensed water to exit the chamber while trapping the hotter steam inside. More advanced autoclaves use a vacuum system to actively pump air out of the chamber before the steam is introduced, ensuring superior steam penetration.
The Safety Net
A safety valve is a critical, non-negotiable component that automatically releases pressure if it exceeds a safe level, preventing a catastrophic failure. A pressure release valve (or whistle) is used for the controlled release of steam at the end of a cycle.
The Waste Management System
A wastewater cooler is often included to cool the discharged water and steam before it enters the facility's drainage system, preventing damage to plumbing.
Understanding the Key Feature Trade-offs
Beyond the basic components, the specific features of an autoclave determine its suitability for different applications.
Gravity vs. Pre-Vacuum Systems
A gravity displacement autoclave relies on the principle that steam is lighter than air. It introduces steam at the top of the chamber, which displaces the heavier, cooler air, forcing it out through a drain at the bottom. This method is simple but less effective for complex loads like porous materials or wrapped instruments.
A pre-vacuum (or pre-vac) autoclave uses a vacuum pump to remove air before steam is injected. This active removal process ensures deep and rapid steam penetration, making it the superior choice for high-throughput labs and complex instrument loads.
Automated vs. Manual Controls
Manual autoclaves require the operator to set and monitor time, temperature, and pressure. Automated systems manage the entire cycle digitally, reducing the chance of human error and providing logs for validation and record-keeping.
Making the Right Choice for Your Goal
The requirements for your autoclave depend entirely on the materials you need to sterilize and the operational demands of your facility.
- If your primary focus is basic sterilization of liquids or simple, unwrapped instruments: A standard gravity displacement autoclave with manual or simple digital controls may be sufficient.
- If your primary focus is high-volume sterilization or processing porous materials (like surgical gowns) and complex instruments: A pre-vacuum system with automated controls is essential for ensuring reliable and validated sterilization.
- If your primary focus is operational safety and process validation: Prioritize an autoclave with advanced safety features, self-diagnostic cycles, and data logging capabilities to meet regulatory standards.
Ultimately, understanding how each component contributes to the core principle of air removal and steam contact will empower you to select the right tool for your specific sterilization needs.
Summary Table:
| Requirement/Component | Key Function |
|---|---|
| Core Principle | Air removal & saturated steam contact |
| Sterilization Conditions | 121°C (250°F) at 15 psi for 30-60 mins |
| Pressure Chamber | Contains the load and maintains pressure |
| Air Removal System | Gravity displacement or pre-vacuum pump |
| Safety Valve | Prevents over-pressurization |
| Control System | Manages temperature, pressure, and cycle time |
Ensure Uncompromising Sterilization in Your Lab
Choosing the right autoclave is critical for the safety and integrity of your work. Whether you process simple liquids or complex, porous materials, KINTEK has the laboratory autoclave solution to meet your specific needs.
Our experts will help you select a system that guarantees reliable, validated sterilization cycles, enhances your lab's efficiency, and complies with industry standards.
Contact KINTEK today for a personalized consultation and ensure your lab achieves sterile confidence.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What type of sterilizer is used for sterilizing liquids? Choose the Right Method for Your Lab
- What is the temperature used for autoclaving of microorganisms? Achieve Reliable Sterilization in Your Lab
- How long is the sterilization cycle in an autoclave? It's More Than Just 15 Minutes
- What is a rotary retort? Achieve Faster, Superior Sterilization for Viscous Foods
- How does a high-pressure hydrothermal autoclave facilitate the synthesis of BiVO4@PANI nanocomposites? Unlock Precision.
- What are the primary functions of high-pressure autoclaves and circulating loop systems in simulating IASCC?
- Why is a Teflon-lined high-pressure autoclave required for Mo-Ti-N catalyst synthesis? Ensure Purity and Precision
- Why are hydrothermal synthesis autoclaves used for mesoporous HA catalysts? Engineering Superior Catalytic Efficiency



















