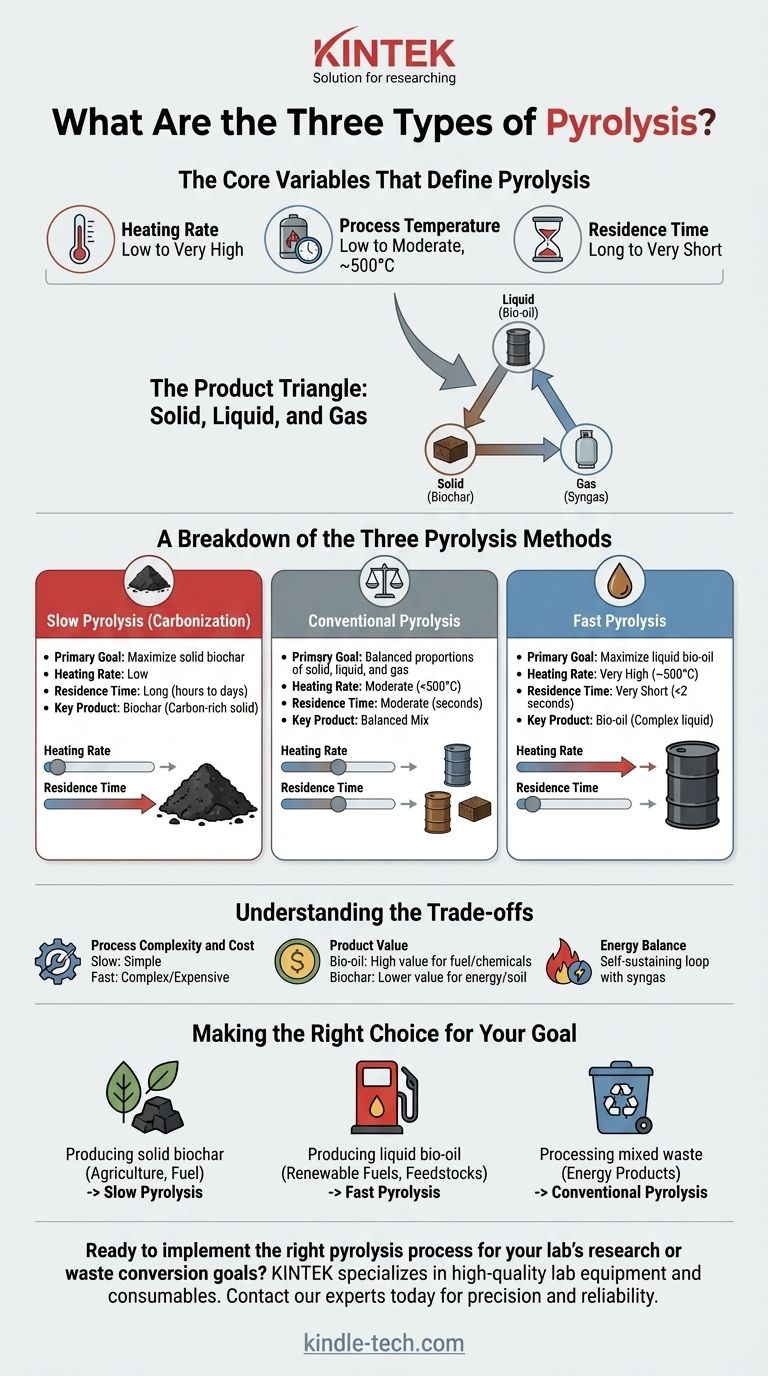
In practice, pyrolysis is categorized into three main types based on the speed and temperature at which material is heated in the absence of oxygen. These process conditions—slow, conventional, and fast—are not arbitrary; they are deliberately controlled to determine whether the final output prioritizes solid char, liquid bio-oil, or a balance of all three products.
The choice between slow, conventional, and fast pyrolysis is a strategic decision driven entirely by the desired end-product. Slower, lower-temperature processes maximize solid biochar, while extremely rapid, moderate-temperature processes maximize liquid bio-oil.

The Core Variables That Define Pyrolysis
To understand the difference between the three methods, you must first understand the key variables that engineers manipulate. The interplay between these factors dictates the chemical reactions that occur and the final product yields.
Heating Rate
The heating rate is the speed at which the feedstock's temperature is increased. This is arguably the most critical parameter, as it determines how quickly volatile compounds are driven out of the material.
Process Temperature
This is the peak temperature the material reaches inside the reactor. Lower temperatures favor the formation of solid char, while moderate temperatures (around 500°C) combined with other factors favor liquid formation.
Residence Time
Residence time refers to how long the material (and its vapor) is held at the peak process temperature. Shorter residence times prevent the initial liquid and gas products from breaking down further into less valuable components.
The Product Triangle: Solid, Liquid, and Gas
These three variables control the proportion of the main outputs:
- Solid: Often called biochar or coke, this carbon-rich solid is what remains of the original feedstock.
- Liquid: Known as bio-oil or pyrolysis oil, this is a complex mixture of condensed organic vapors.
- Gas: A mix of non-condensable gases (like hydrogen, carbon monoxide, and methane), often called syngas.
A Breakdown of the Three Pyrolysis Methods
Each type of pyrolysis represents a specific point on a spectrum, optimized for a particular product.
Slow Pyrolysis (Carbonization)
The primary goal of slow pyrolysis is to maximize the yield of solid biochar. This is the oldest and simplest form of the technology, historically used to make charcoal from wood.
It is defined by a very low heating rate, a relatively low peak temperature (often below 400°C), and a long residence time that can last for hours or even days. This slow "cooking" process drives off moisture and volatiles, leaving a stable, carbon-dense solid.
Conventional Pyrolysis
Conventional pyrolysis serves as a middle ground, producing more balanced proportions of solid, liquid, and gas. It is not optimized for a single output, making it a flexible option for general waste processing.
This method uses a slower heating rate than fast pyrolysis but faster than slow pyrolysis, with temperatures typically below 500°C. The vapor residence time is moderate, usually in the range of several seconds, allowing for some secondary cracking of the vapors to occur.
Fast Pyrolysis
The sole objective of fast pyrolysis is to maximize the yield of liquid bio-oil. This requires highly controlled and sophisticated engineering.
It is characterized by an extremely high heating rate and a very short residence time for vapors (typically less than 2 seconds). The material is quickly heated to a moderate temperature (around 500°C) to break it down into vapors, which are then rapidly cooled and condensed into liquid bio-oil before they can break down further into gas.
Understanding the Trade-offs
Choosing a pyrolysis method involves balancing technical complexity, cost, and the value of the desired product.
Process Complexity and Cost
Slow pyrolysis can be achieved with simple technology like fixed-bed or batch reactors (kilns). Fast pyrolysis, however, requires advanced reactors like fluidized-beds or ablative systems to achieve the necessary rapid heat transfer, making it more complex and expensive to build and operate.
Product Value
Liquid bio-oil produced from fast pyrolysis can be refined into transportation fuels or used as a source for specialty chemicals, giving it a potentially high market value. Biochar from slow pyrolysis is primarily used for energy, as a soil amendment, or for producing activated carbon, which often represents a lower-value application.
Energy Balance
All pyrolysis processes require a significant energy input to reach operating temperature. A well-designed plant will use the non-condensable syngas it produces as a fuel source, creating a self-sustaining thermal loop that minimizes external energy needs.
Making the Right Choice for Your Goal
Your application's goal dictates the correct pyrolysis method.
- If your primary focus is producing solid biochar for agriculture or charcoal for fuel: Slow pyrolysis is the most direct and cost-effective method.
- If your primary focus is producing liquid bio-oil for renewable fuels or chemical feedstocks: Fast pyrolysis is the necessary choice, despite its higher technical complexity.
- If your primary focus is processing mixed waste into a balanced slate of energy products: Conventional pyrolysis offers a robust and flexible solution.
Ultimately, selecting the right process is about matching the technology to the value you intend to create from your feedstock.
Summary Table:
| Type of Pyrolysis | Primary Goal | Heating Rate | Residence Time | Key Product |
|---|---|---|---|---|
| Slow Pyrolysis | Maximize solid biochar | Low | Long (hours) | Biochar |
| Conventional Pyrolysis | Balanced solid, liquid, gas | Moderate | Moderate (seconds) | Balanced Mix |
| Fast Pyrolysis | Maximize liquid bio-oil | Very High | Very Short (<2 seconds) | Bio-oil |
Ready to implement the right pyrolysis process for your lab's research or waste conversion goals?
KINTEK specializes in providing high-quality lab equipment and consumables for advanced thermal processes. Whether you are developing biochar applications, optimizing bio-oil production, or processing mixed waste streams, our reactors and systems are designed for precision and reliability.
Contact our experts today to discuss how our solutions can help you achieve superior yields and efficiency in your pyrolysis projects.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Wall Mounted Water Distillation Unit
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What role does a high-pressure reactor play in the hydrodeoxygenation (HDO) of bio-oil? Drive Deep Fuel Upgrading
- Why must SCWG reactors maintain a specific heating rate? Protect Your High-Pressure Vessels from Thermal Stress
- Why are high-strength alloy tube reactors critical for HHIP? Ensuring Safety and Purity in High-Pressure Environments
- Why use high-pressure reactors for food waste pretreatment? Boost Hydrogen Production Efficiency Today!
- What is the advantage of using high-pressure hydrothermal reactors to treat biomass waste? Efficient Resource Recovery



















