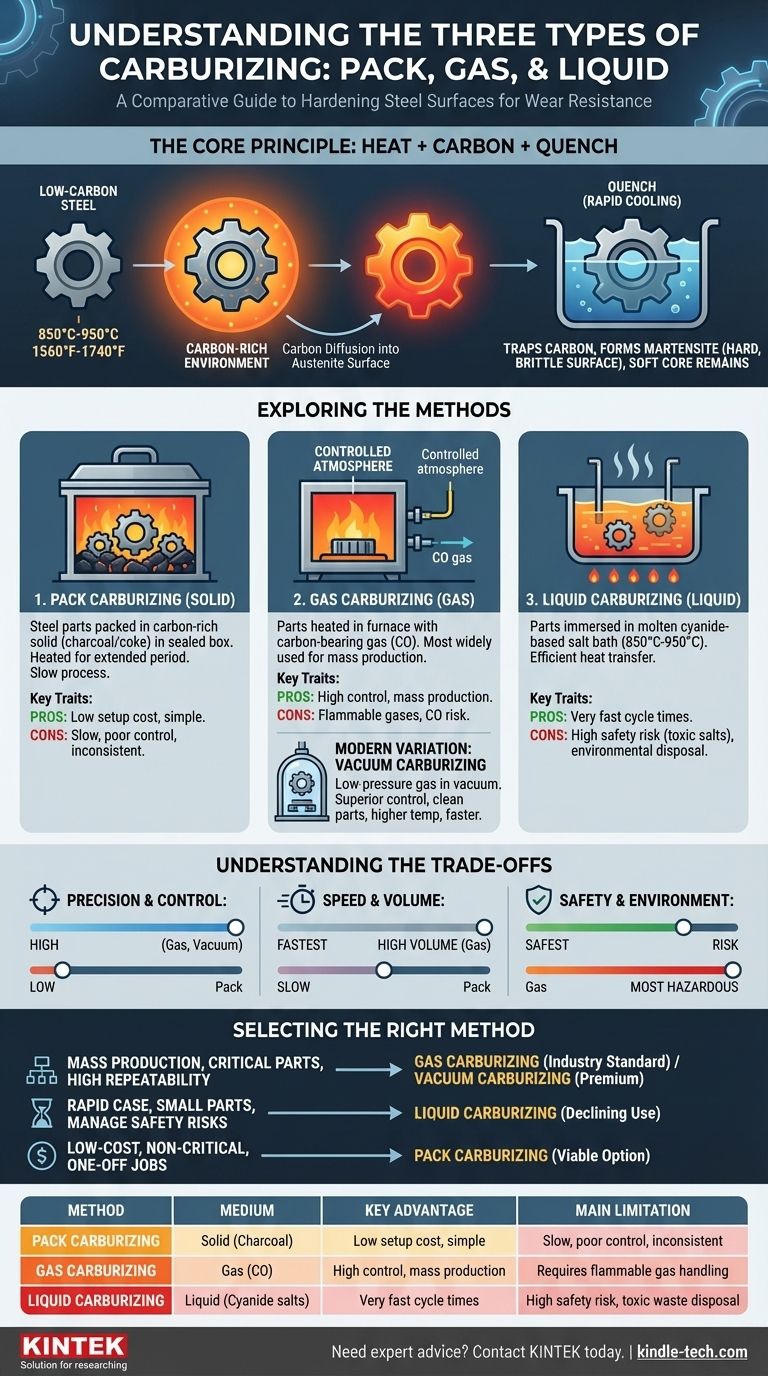
In practice, there are three primary methods of carburizing based on the medium used to introduce carbon into the steel: pack carburizing (solid), gas carburizing (gas), and liquid carburizing (liquid). Each process involves heating the steel in the presence of a carbon-rich material, allowing carbon atoms to diffuse into the surface. The true hardening, however, only occurs after the component is quenched, which locks the diffused carbon into the steel's crystal structure.
While all carburizing methods aim to create a hard, wear-resistant surface on a softer, ductile core, the choice of method is a critical engineering decision. It requires balancing the need for process control, cost, production volume, and significant safety considerations.

The Core Principle: How Carburizing Works
Carbon Diffusion at High Temperature
Carburizing is a heat treatment process performed on low-carbon steels. The parts are heated to a high temperature, typically between 850°C and 950°C (1560°F to 1740°F), within a carbon-rich environment.
At this elevated temperature, the steel's crystal structure changes to austenite, which has a high solubility for carbon. This allows carbon atoms from the surrounding environment to diffuse into the surface of the part.
The Quench and Harden Cycle
The diffusion process alone only enriches the surface with carbon; it does not make it hard. After the part has absorbed the desired amount of carbon to the required depth, it is rapidly cooled, or quenched.
This rapid cooling traps the carbon atoms in the steel's structure, creating a very hard, brittle phase known as martensite on the surface. The core, which has a lower carbon content, remains softer and tougher, resulting in a component with excellent wear resistance and fatigue life.
Exploring the Primary Carburizing Methods
The fundamental difference between the three main types of carburizing lies in the source of the carbon—whether it comes from a solid, a gas, or a liquid.
1. Pack Carburizing (Solid Medium)
This is the oldest and simplest method. Steel parts are packed into a sealed steel box, surrounded by a carbon-rich solid compound, typically charcoal or coke mixed with an energizer like barium carbonate.
The box is heated in a furnace for an extended period, allowing carbon monoxide gas generated by the compound to transfer carbon to the steel. This method is slow and provides limited control over the case depth and carbon concentration.
2. Gas Carburizing (Gas Medium)
Gas carburizing is the most widely used method in modern industry due to its process control and suitability for mass production. Parts are heated in a furnace with a tightly controlled atmosphere.
A carbon-bearing gas (an "endothermic" gas) rich in carbon monoxide (CO) is introduced. This gas serves as the primary source for the carbon that diffuses into the steel. By precisely managing gas composition, temperature, and time, engineers can achieve highly consistent and predictable case depths.
A Modern Variation: Vacuum Carburizing
Vacuum carburizing, also known as "low-pressure carburizing," is an advanced form of gas carburizing. The process starts by heating the parts in a vacuum to clean the surface. Then, a pure hydrocarbon gas like acetylene or propane is introduced at low pressure.
This method offers superior control, produces exceptionally clean parts, and allows for even higher processing temperatures, which can shorten cycle times. It eliminates the risk of surface oxidation entirely.
3. Liquid Carburizing (Liquid Medium)
In this method, steel parts are immersed in a molten bath of carbon-rich salt, typically a cyanide-based compound, operating at 850°C to 950°C.
Liquid carburizing is very fast due to the efficient heat transfer from the liquid to the metal parts. It can produce a hard case in a much shorter time than pack or gas carburizing. However, the use of toxic cyanide salts presents significant safety hazards and environmental disposal challenges.
Understanding the Trade-offs
Choosing a carburizing method involves navigating a series of compromises between precision, cost, and safety. No single method is universally superior.
Precision and Control
Vacuum and Gas carburizing offer the highest degree of control. Computerized furnace controls allow for precise management of the carbon potential, ensuring repeatable results for critical components like gears and bearings.
Liquid carburizing offers good uniformity but less dynamic control over the carbon gradient compared to gas processes. Pack carburizing provides the least amount of control and is prone to inconsistent results.
Speed, Cost, and Volume
For high-volume production, gas carburizing is often the most cost-effective solution, balancing throughput and control.
Liquid carburizing offers the fastest cycle times, which can be advantageous for smaller parts, but the high costs associated with handling and disposing of hazardous salts must be factored in.
Pack carburizing has a low initial setup cost and is useful for one-off jobs or very large components where building a controlled atmosphere furnace is impractical.
Safety and Environmental Impact
This is a critical differentiator. Liquid carburizing is by far the most hazardous due to the extreme toxicity of the cyanide salts used. It requires stringent safety protocols and specialized waste management.
Gas carburizing involves handling flammable gases and the risk of carbon monoxide poisoning, requiring robust ventilation and safety interlocks. Vacuum carburizing is the safest, as it operates in a sealed chamber and eliminates the risks associated with a CO-rich atmosphere.
Selecting the Right Method for Your Application
Your choice must be driven by the specific requirements of the component and your production environment.
- If your primary focus is mass production of critical parts with high repeatability: Gas carburizing is the industry standard, with vacuum carburizing being the premium choice for the highest quality and cleanliness.
- If your primary focus is rapid case hardening for small to medium-sized parts and you can manage the safety risks: Liquid carburizing offers unmatched speed, but its use is declining due to environmental and safety concerns.
- If your primary focus is low-cost treatment for non-critical parts or single-piece jobs: Pack carburizing is a viable, though technically inferior, option that gets the job done without complex equipment.
Ultimately, understanding the strengths and weaknesses of each carburizing method empowers you to select the process that delivers the required performance at an acceptable cost and risk.
Summary Table:
| Method | Medium | Key Advantage | Main Limitation |
|---|---|---|---|
| Pack Carburizing | Solid (Charcoal) | Low setup cost, simple | Slow, poor control, inconsistent results |
| Gas Carburizing | Gas (CO) | High control, ideal for mass production | Requires flammable gas handling |
| Liquid Carburizing | Liquid (Cyanide salts) | Very fast cycle times | High safety risk, toxic waste disposal |
Need expert advice on selecting the right carburizing method for your lab or production line? KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our team can help you choose the optimal heat treatment process to achieve superior hardness, wear resistance, and component longevity. Contact us today to discuss your specific requirements and enhance your manufacturing efficiency!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the application of quenching oil? Achieve Superior Hardness and Durability in Metal Parts
- How does an industrial high-temperature steam oxidation device ensure representative results? Simulating Reactor Safety
- What happens if proper clearance is not maintained between joints while brazing? Avoid Common Joint Failures
- How is a high-temperature furnace utilized for SAPO-34 membrane alumina supports? Achieve 950°C Precision
- What are the methods used in leak hunting in the vacuum system? Find & Fix Leaks Efficiently
- Why is post-treatment in a furnace required after hydrothermal synthesis of Magnéli phase? Ensure Material Stability
- What is the process of sintering? A Guide to Powder-Based Manufacturing
- What are the types of filler metal in brazing? Select the Right Alloy for a Strong, Durable Joint



















