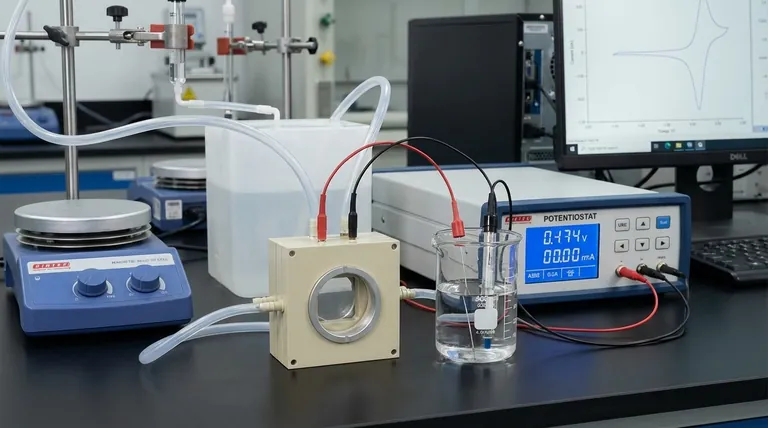
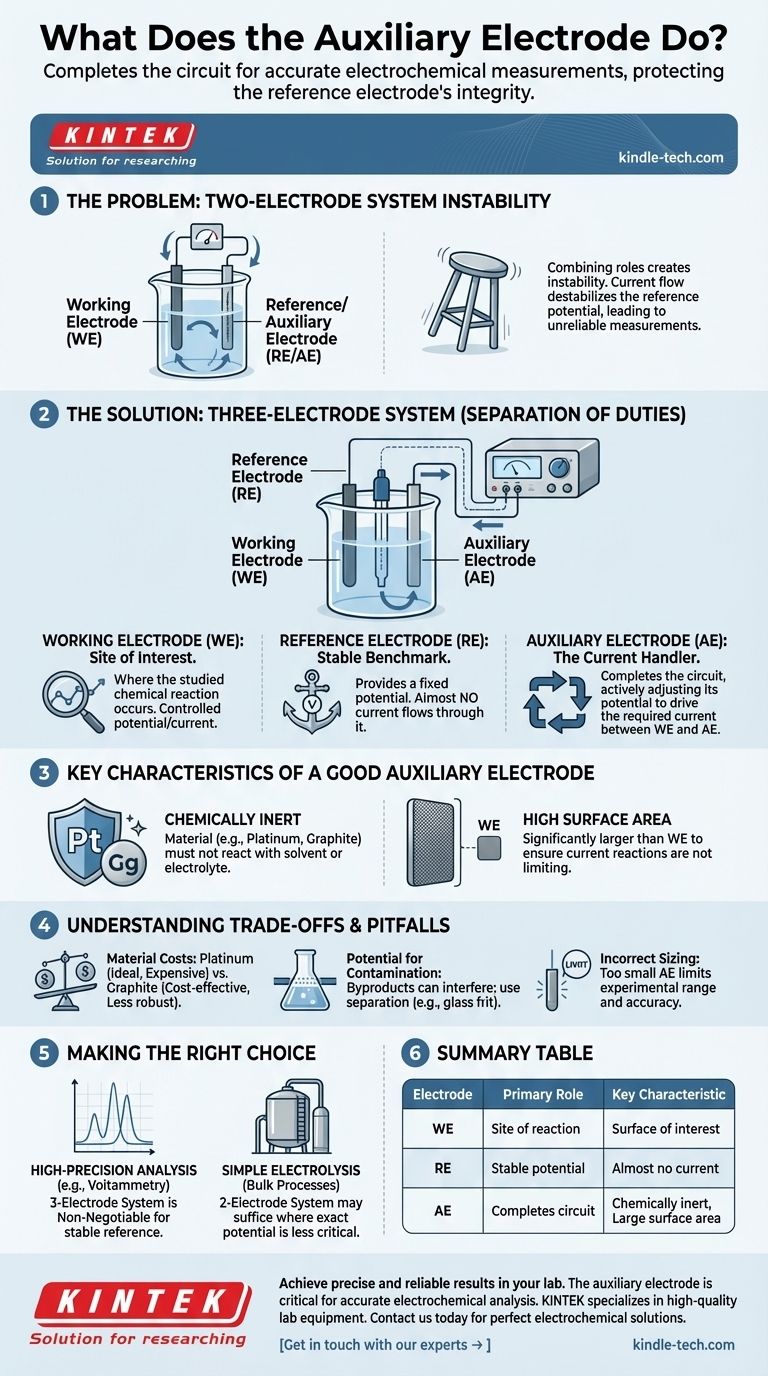
In any electrochemical experiment involving current, the auxiliary electrode (also called a counter electrode) serves one primary purpose: to complete the electrical circuit. It works in tandem with the working electrode, allowing current to flow so that the reaction of interest can be driven and studied, all while ensuring the measurement remains stable and accurate.
The auxiliary electrode's true function is to protect the integrity of your measurement. By handling the entire flow of current, it isolates the reference electrode, ensuring the potential at the working electrode is measured against a stable, undisturbed benchmark.
The Problem with a Two-Electrode System
To understand why an auxiliary electrode is necessary, we must first look at the limitations of a simpler setup. In many situations, a two-electrode system is insufficient for precise control and measurement.
Combining Roles Creates Instability
In a two-electrode cell, you have only a working electrode and a second electrode that is forced to act as both the reference (the stable point against which potential is measured) and the auxiliary (the path for current).
The Impact of Current Flow
The potential of a reference electrode is only stable when no significant current passes through it. Forcing it to also serve as the current-carrying counter electrode destabilizes its potential. This makes it impossible to know the true potential at your working electrode, rendering your measurements unreliable.
The Three-Electrode Solution: A Separation of Duties
The introduction of a third electrode—the auxiliary electrode—solves this problem by creating a clear separation of roles within the electrochemical cell. This setup, managed by an instrument called a potentiostat, is the standard for modern electroanalysis.
The Working Electrode (WE): The Site of Interest
This is where the chemical reaction you want to study occurs. Your entire experiment is designed to control and measure the potential and/or current at this surface.
The Reference Electrode (RE): The Stable Benchmark
The reference electrode's sole job is to provide a fixed, known potential. The potentiostat measures the potential difference between the working electrode and the reference electrode. Crucially, almost no current flows through the RE, preserving its stability.
The Auxiliary Electrode (AE): The Current Handler
The auxiliary electrode completes the circuit. The potentiostat applies current between the working electrode and the auxiliary electrode. The AE's potential is actively adjusted by the instrument to whatever voltage is needed to balance the reaction at the WE and allow the target current to flow. It effectively absorbs the electrical stress of the system.
Key Characteristics of a Good Auxiliary Electrode
To perform its role effectively, an auxiliary electrode should have specific properties that prevent it from interfering with the measurement or limiting the experiment.
Chemically Inert
The auxiliary electrode must be made from a material, like platinum or graphite, that will not react with your solvent or electrolyte. Its only job is to facilitate electron transfer, not to become part of the chemistry you are studying.
High Surface Area
The surface area of the auxiliary electrode should be significantly larger than that of the working electrode. This ensures that the reactions happening at the auxiliary surface can easily keep up with the current demands of the working electrode, preventing the AE from becoming the limiting factor in your experiment.
Understanding the Trade-offs and Pitfalls
While essential, the auxiliary electrode is not a "set and forget" component. Proper selection and placement are critical for good data.
Material Costs
Platinum is an ideal material due to its inertness and efficiency, but it is expensive. Graphite is a common, cost-effective alternative, but it can be less robust and its surface can change over time.
Potential for Contamination
The reactions occurring at the auxiliary electrode (often the oxidation or reduction of the solvent) produce chemical byproducts. In a poorly designed cell, these products can diffuse to the working electrode and interfere with your reaction of interest. This is why separation, sometimes with a glass frit, is important.
Incorrect Sizing
Using an auxiliary electrode that is too small is a common mistake. If its surface area is insufficient, it cannot pass the required current efficiently. This will limit the experimental range and can lead to inaccurate results, as the instrument struggles to maintain the target potential at the working electrode.
Making the Right Choice for Your Goal
The need for an auxiliary electrode is directly tied to the need for accurate potential control.
- If your primary focus is high-precision analysis (e.g., voltammetry): A three-electrode system with a distinct, inert auxiliary electrode is non-negotiable for achieving a stable reference potential.
- If your primary focus is simple electrolysis or bulk processes: A two-electrode system may suffice where the exact potential is less critical than driving the overall reaction with a large current.
- If you are designing a new experiment: Always default to a three-electrode setup. Using a properly sized auxiliary electrode ensures that the data you collect is a true reflection of the chemistry at your working electrode.
Ultimately, the auxiliary electrode is the unsung hero that enables precise and repeatable electrochemical control.
Summary Table:
| Electrode | Primary Role | Key Characteristic |
|---|---|---|
| Working Electrode (WE) | Site of the chemical reaction being studied. | The surface of interest for the experiment. |
| Reference Electrode (RE) | Provides a stable, known potential for measurement. | Almost no current flows through it. |
| Auxiliary Electrode (AE) | Completes the electrical circuit by handling current flow. | Chemically inert (e.g., Platinum) and has a large surface area. |
Achieve precise and reliable results in your lab. The auxiliary electrode is critical for accurate electrochemical analysis. For your research to be trustworthy, you need the right equipment.
KINTEK specializes in high-quality lab equipment and consumables, including electrochemical cells and components. We provide the reliable tools your laboratory needs to ensure measurement integrity and experimental success.
Contact us today to find the perfect electrochemical solutions for your specific application. Get in touch with our experts →
Visual Guide
Related Products
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Button Battery Case for Battery Lab Applications
People Also Ask
- Which parameters must be strictly controlled using an all-PTFE electrolytic cell? Ensure Precision and Safety
- What are the primary functions of a high-performance electrolytic cell in the eCO2R process? Optimize Your Lab Results
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What role does a two-electrode electrochemical reactor play in TiO2 growth? Achieve Ordered Nanostructures Today
- What are the advantages of using a PTFE deposition tank for EPD? Achieve Unmatched Coating Precision on Stainless Steel