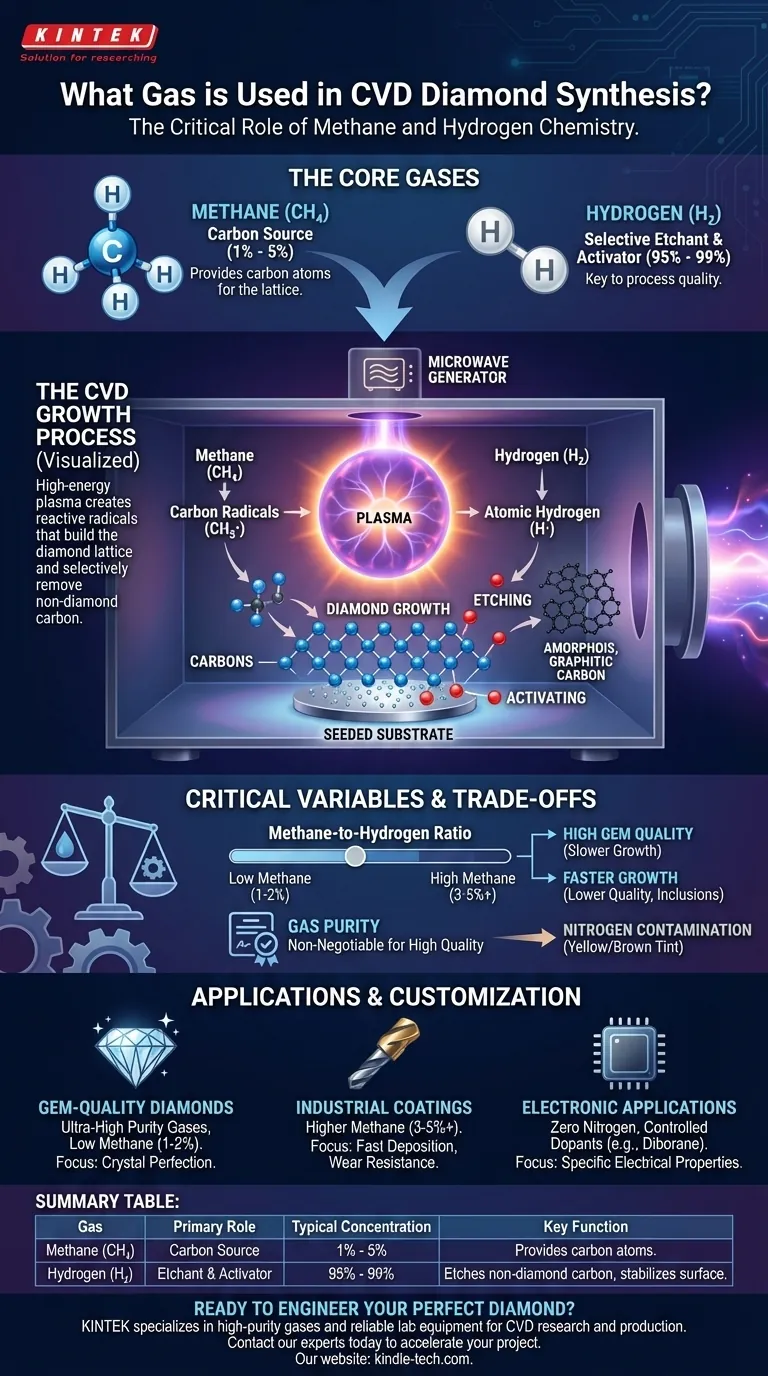
In chemical vapor deposition (CVD) for diamond synthesis, the process fundamentally relies on a carefully controlled mixture of a carbon-source gas and an etchant gas. The most common combination is a small percentage of methane (CH₄), which provides the carbon atoms, mixed with a vast excess of hydrogen (H₂) gas.
Creating a synthetic diamond is not a simple act of depositing carbon. The process requires a precise, high-energy environment where methane supplies the carbon building blocks, while a super-heated hydrogen plasma simultaneously and selectively removes any non-diamond carbon, ensuring only the desired crystal structure can form and grow.

The Roles of Methane and Hydrogen
The choice of methane and hydrogen is not arbitrary; each performs a distinct and critical function in the reaction chamber. The balance between them is the single most important factor in determining the quality and growth rate of the diamond.
The Carbon Source: Methane (CH₄)
Methane serves as the raw material for the diamond. When subjected to high energy (typically from microwaves or hot filaments), the methane molecules break apart into various carbon-containing radicals, such as CH₃·.
These highly reactive fragments are the "building blocks" that eventually attach to the diamond seed crystal to grow the lattice, atom by atom. While other hydrocarbons can be used, methane is favored for its simplicity, purity, and control.
The Selective Etchant: Hydrogen (H₂)
Hydrogen's role is far more complex and is the true key to the process. In the high-energy plasma, molecular hydrogen (H₂) is split into atomic hydrogen (H·), which is intensely reactive. This atomic hydrogen performs two vital jobs.
First, it aggressively etches away undesirable forms of carbon. During deposition, both diamond (sp³-bonded) and graphite/amorphous carbon (sp²-bonded) can form. Atomic hydrogen removes the unstable, graphitic carbon much faster than it removes the stable diamond carbon, effectively cleaning the growth surface.
Second, it activates the growth surface. Atomic hydrogen terminates the "dangling bonds" on the diamond surface, stabilizing it and creating specific active sites where carbon radicals from the methane can attach and successfully continue the diamond lattice structure.
The CVD Growth Environment
The gases alone are inert. They must be combined with a specific substrate and activated with immense energy within a controlled environment to initiate diamond growth.
Creating the Plasma
To break down the stable methane and hydrogen gases, a massive amount of energy is required to create a plasma. This is most commonly achieved using microwaves to generate a ball of glowing plasma inside the vacuum chamber.
This plasma, reaching thousands of degrees Celsius, provides the energy needed to create the atomic hydrogen and carbon radicals that drive the entire reaction.
Seeding the Substrate
Diamond cannot grow on just any surface. The process begins with a substrate, often a small, flat disc of silicon. This substrate is "seeded" by polishing it with microscopic diamond dust.
These tiny diamond crystals act as the nucleation points, or seeds, upon which the carbon atoms from the gas phase will align and begin to build a new, larger diamond layer.
Understanding the Trade-offs and Variables
Controlling the gas chemistry is a game of precision. Minor deviations can drastically alter the outcome, leading to poor-quality diamond or no growth at all.
The Critical Methane-to-Hydrogen Ratio
The concentration of methane in the hydrogen gas is a master variable. A typical ratio is very low, often between 1% and 5% methane.
Increasing the methane percentage can speed up the growth rate, but it risks overwhelming the hydrogen's ability to etch away graphite. This results in a lower-quality diamond with dark inclusions and internal stress. For high-purity gems, the ratio is kept very low.
Gas Purity and Contamination
The purity of the source gases is non-negotiable for producing high-quality diamonds. Even trace amounts of nitrogen in the chamber can be incorporated into the diamond lattice, giving it an undesirable yellow or brown tint.
For electronic-grade diamond, where electrical properties are paramount, controlling for unwanted elements like nitrogen and intentionally adding dopants like boron is a primary focus.
Making the Right Choice for Your Goal
The specific gas mixture and process parameters are always tuned to the desired properties of the final diamond.
- If your primary focus is gem-quality, colorless diamonds: You must use ultra-high purity gases with a low methane concentration (1-2%) to prioritize crystal perfection and clarity over growth speed.
- If your primary focus is industrial coatings for wear resistance: You can use a higher methane concentration (3-5% or more) to achieve a faster deposition rate, as minor graphitic inclusions are less critical than overall hardness and thickness.
- If your primary focus is advanced electronic applications: You must strictly eliminate nitrogen and may introduce precisely metered dopant gases, such as diborane (for boron doping), to engineer specific semiconductor properties.
Ultimately, mastering the gas chemistry is the foundation for engineering a synthetic diamond with the exact properties required for its intended application.
Summary Table:
| Gas | Primary Role | Typical Concentration | Key Function |
|---|---|---|---|
| Methane (CH₄) | Carbon Source | 1% - 5% | Provides carbon atoms to build the diamond lattice. |
| Hydrogen (H₂) | Selective Etchant & Activator | 95% - 99% | Etches non-diamond carbon and stabilizes the growth surface. |
Ready to engineer your perfect diamond?
The precise gas chemistry detailed here is the foundation of successful CVD diamond synthesis. Whether your goal is creating flawless gemstones, ultra-hard industrial coatings, or advanced semiconductor components, the right equipment and consumables are critical.
KINTEK specializes in lab equipment and consumables, serving all your laboratory needs for CVD research and production. We provide the high-purity gases and reliable systems you need to control every variable and achieve consistent, high-quality results.
Contact our experts today to discuss how we can support your specific diamond synthesis goals and accelerate your project from concept to creation.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings



















