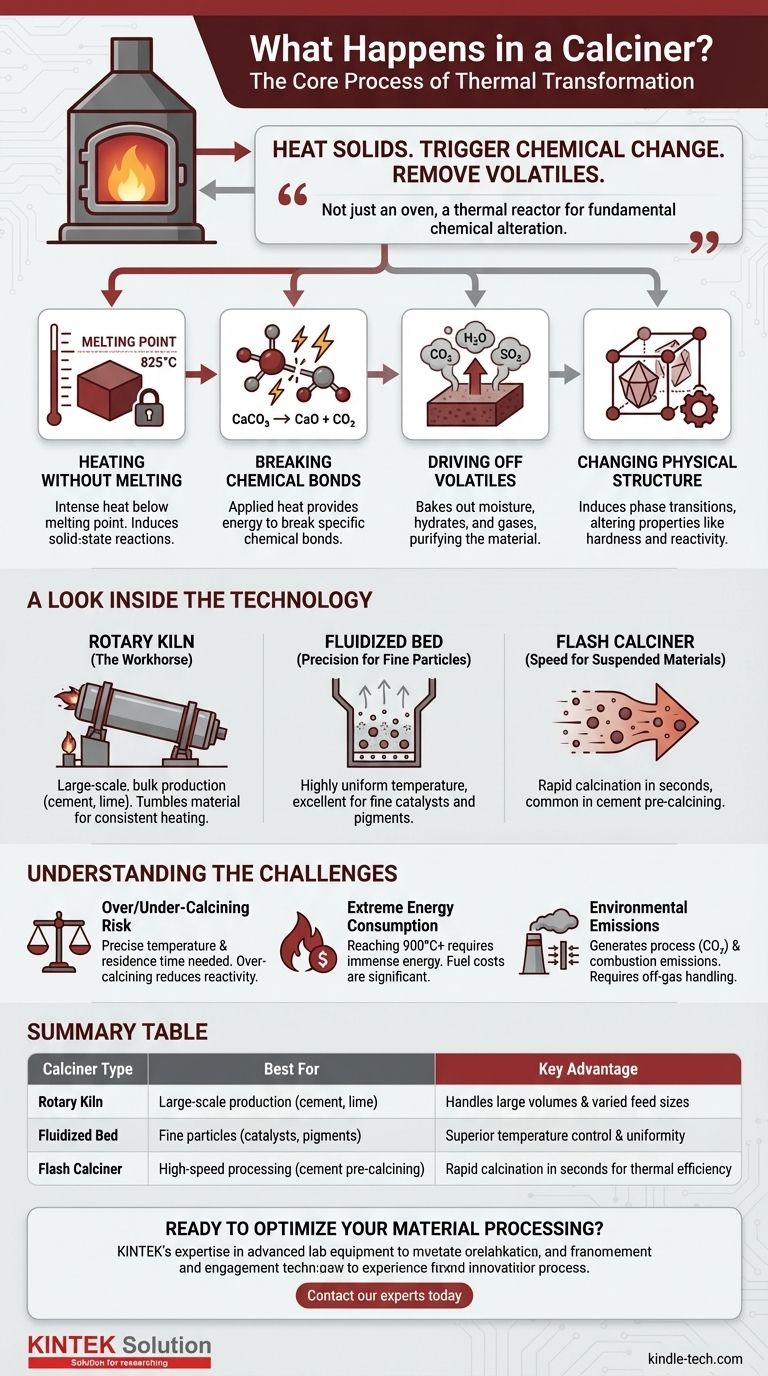
In short, a calciner is a high-temperature industrial furnace that heats solid materials to trigger a chemical change or remove volatile substances. It works by raising a material's temperature to a precise point below its melting point, causing it to decompose, release trapped water or gases like carbon dioxide, and transform its fundamental chemical and physical structure.
A calciner is not simply an oven for drying; it is a thermal reactor. Its primary purpose is to use carefully controlled heat to fundamentally alter a material's chemical composition, driving off specific components to create a new, desired substance.

The Core Process: A Chemical Transformation
At its heart, calcination is a process of controlled thermal decomposition. It breaks down complex compounds into simpler, more stable ones by applying immense heat in a controlled atmosphere, which typically has a limited supply of oxygen.
Heating Without Melting
The first principle of calcination is to heat the material intensely but to keep it below its melting point. The goal is to induce chemical reactions within the solid-state, not to liquefy it.
This precise temperature control is critical for achieving the desired outcome.
Breaking Chemical Bonds
The applied heat provides the energy needed to break specific chemical bonds within the material. This is the core of the transformation.
A classic example is the production of lime from limestone. Limestone (calcium carbonate, CaCO₃) is heated to over 825°C (1517°F), causing it to decompose into lime (calcium oxide, CaO) and carbon dioxide (CO₂). The CO₂ gas is driven off, leaving the transformed solid behind.
Driving Off Volatiles
Many materials contain volatile substances that must be removed. These can include physically trapped moisture, chemically bound water (hydrates), or gases like carbon dioxide and sulfur dioxide.
The calcination process effectively "bakes out" these components, purifying the material and changing its properties. For example, bauxite ore is calcined to remove water and produce alumina, the primary ingredient for making aluminum.
Changing Physical Structure
Beyond chemical changes, calcination can also induce phase transitions, altering the material's crystalline structure. This can change properties like hardness, reactivity, and surface area, making the final product suitable for specific industrial applications.
A Look Inside the Technology
While the principle is universal, different types of calciners are engineered for different materials and production scales. The choice of technology is critical for efficiency and product quality.
The Rotary Kiln: The Industrial Workhorse
This is the most common type of calciner. It is a large, rotating, cylindrical steel tube lined with refractory brick and mounted at a slight incline.
Material is fed into the higher end and slowly tumbles its way to the lower end as the kiln rotates. This tumbling action ensures consistent mixing and exposure to heat, which is typically provided by a large burner at the discharge end.
The Fluidized Bed: Precision for Fine Particles
In a fluidized bed calciner, hot gas is forced upwards through a bed of fine-particulate material. This gas flow causes the solids to become suspended and behave like a boiling liquid.
This "fluidization" results in extremely efficient heat transfer and highly uniform temperature control, making it ideal for processes where precision is paramount, such as in catalyst and pigment manufacturing.
The Flash Calciner: Speed for Suspended Materials
Flash calciners are used for very fine materials that can be transported in a hot gas stream. The particles are calcined in a matter of seconds as they are carried through the system. This method is common in the pre-calcining stage of modern cement production.
Understanding the Trade-offs and Challenges
Calcination is a powerful but demanding industrial process with significant challenges that must be managed for successful operation.
The Risk of Over- or Under-Calcining
Achieving the correct final product requires a precise balance of temperature and residence time.
Heating for too long or at too high a temperature can lead to over-calcining or "dead-burning," which sinters the material and drastically reduces its chemical reactivity. Conversely, insufficient heat or time results in under-calcining, an incomplete reaction that leaves impurities in the final product.
Extreme Energy Consumption
Bringing materials to temperatures often exceeding 900°C (1650°F) requires a tremendous amount of energy. Fuel costs are a major operational expense, and process efficiency is a constant focus of engineering and design improvements.
Environmental and Emissions Control
Calcination generates significant emissions. This includes process emissions (like the CO₂ released from limestone) and combustion emissions (from burning fuel). Modern plants require extensive off-gas handling systems, including cyclones and baghouses for dust control and sometimes scrubbers, to meet environmental regulations.
Making the Right Choice for Your Goal
Selecting the appropriate calciner technology depends entirely on the material properties and the desired outcome.
- If your primary focus is large-scale, robust production of bulk materials like cement or lime: The rotary kiln is the industry standard due to its ability to handle large volumes and a wide variety of feed material sizes.
- If your primary focus is achieving highly uniform product quality with fine particles, like for catalysts or pigments: A fluidized bed calciner is the superior choice, offering unparalleled temperature control and heat transfer efficiency.
- If your primary focus is integrating the process into a larger system for maximum thermal efficiency, as in modern cement plants: A precalciner or flash calciner system is designed to use waste heat and dramatically improve overall energy performance.
By mastering this thermal transformation process, we can convert raw, abundant minerals into the foundational building blocks of modern industry.
Summary Table:
| Calciner Type | Best For | Key Advantage |
|---|---|---|
| Rotary Kiln | Large-scale production (cement, lime) | Handles large volumes and varied feed sizes |
| Fluidized Bed | Fine particles (catalysts, pigments) | Superior temperature control and uniformity |
| Flash Calciner | High-speed processing (cement pre-calcining) | Rapid calcination in seconds for thermal efficiency |
Ready to optimize your material processing with precision thermal technology?
At KINTEK, we specialize in advanced lab equipment and consumables for industrial research and development. Whether you are developing new catalysts, refining minerals, or scaling up a production process, our expertise in thermal processing can help you achieve superior product quality and efficiency.
Let's discuss your specific calcination needs and explore how our solutions can benefit your laboratory or pilot plant. Contact our experts today for a personalized consultation.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the temperature of a carbon regeneration kiln? Mastering the 750-800°C Reactivation Process
- What is the temperature for activated carbon regeneration? Key Ranges from 220°C to 900°C
- Can you restore activated carbon? Understanding the Industrial Reactivation Process
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What temperature is a carbon regeneration kiln? Master the 650°C-800°C Range for Optimal Results



















