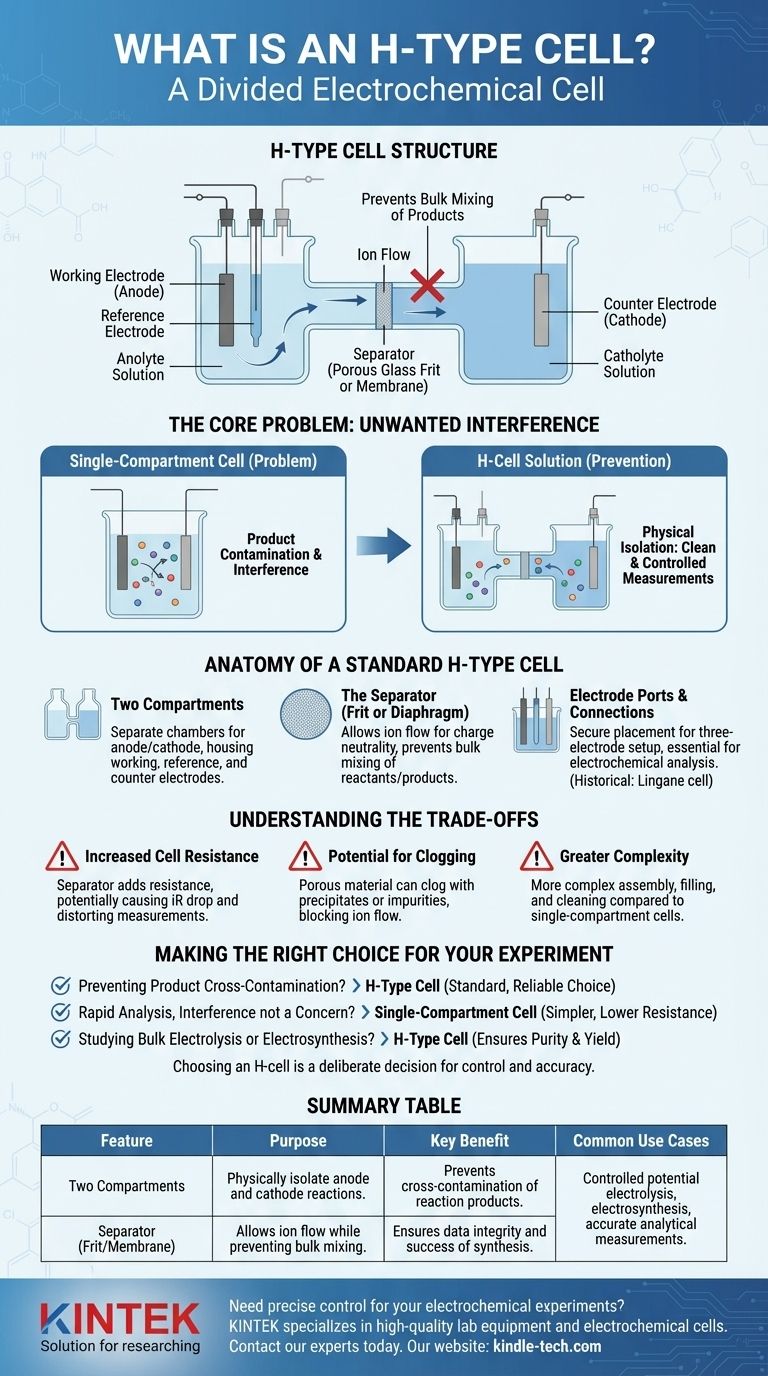
In essence, an H-type cell is a divided electrochemical cell named for its resemblance to the letter 'H'. It consists of two separate compartments, typically for the anode and cathode, which are connected by a bridge containing a separator like a porous glass frit or membrane. This fundamental design allows for the physical isolation of the two electrode reactions.
The central purpose of an H-type cell is to prevent the products generated at one electrode from migrating to and interfering with the reactions occurring at the other electrode. This separation is critical for achieving clean, controlled, and accurate electrochemical measurements.

The Core Problem H-Cells Solve: Unwanted Interference
In many electrochemical experiments, the products formed at the working electrode (anode) can be reactive. If these products diffuse to the counter electrode (cathode), they can be consumed or cause side reactions, compromising the integrity of the experiment.
Preventing Cross-Contamination
The H-cell's divided structure acts as a physical barrier. It stops the bulk mixing of the solutions (anolyte and catholyte) in each compartment.
This ensures that the species being studied at the working electrode are not consumed or altered by byproducts generated at the counter electrode, leading to more reliable data.
Isolating Electrode Processes
By keeping the two half-cells separate, researchers can study a specific electrode process in a pristine environment.
This is essential for applications like controlled potential electrolysis, where the goal is to exhaustively convert a reactant into a product at one electrode without interference from the other.
Anatomy of a Standard H-Type Cell
While designs vary, most H-type cells share three fundamental components that enable their function.
The Two Compartments
The cell is composed of two distinct glass chambers or arms. One chamber houses the working electrode and the reference electrode, while the other contains the counter electrode.
The Separator (Frit or Diaphragm)
Connecting the two compartments is a bridge that contains a separator. This is often a porous glass frit or an ion-exchange membrane.
The separator's role is crucial: it allows ions to flow between the compartments to maintain charge neutrality and complete the electrical circuit, but it prevents the bulk mixing of the larger reactant and product molecules.
Electrode Ports and Connections
Each compartment has openings (ports) to allow for the secure placement of the electrodes. Modern H-cells are often part of a three-electrode setup, which is the standard for most electrochemical analysis.
A specific variation mentioned in historical texts is the Lingane cell, which was an H-type design adapted for use with mercury pool electrodes.
Understanding the Trade-offs
The benefits of isolation come with certain practical considerations that must be managed.
Increased Cell Resistance
The separator, by its very nature, adds resistance to the flow of ions. This increased overall cell resistance can lead to a larger iR drop (a voltage drop due to resistance), which can distort electrochemical measurements, particularly at high currents.
Potential for Clogging
The porous material of the frit can become clogged over time by reaction products, precipitates, or impurities in the solvent. This increases resistance further and can eventually block ion flow entirely.
Greater Complexity
Compared to a simple single-compartment beaker cell, the H-type cell is more complex to assemble, fill, and clean. This adds a small overhead to the experimental setup time.
Making the Right Choice for Your Experiment
Selecting the correct cell geometry is fundamental to good experimental design in electrochemistry.
- If your primary focus is preventing product cross-contamination: The H-type cell is the standard and most reliable choice for your experiment.
- If your primary focus is rapid analysis where interference is not a concern: A simpler, single-compartment cell is often more efficient and provides lower cell resistance.
- If your primary focus is studying bulk electrolysis or electrosynthesis: An H-type cell is almost always necessary to ensure product purity and high reaction yield.
Ultimately, choosing an H-type cell is a deliberate decision to prioritize control and accuracy over simplicity and speed.
Summary Table:
| Feature | Purpose |
|---|---|
| Two Compartments | Physically isolate the anode and cathode reactions. |
| Separator (Frit/Membrane) | Allows ion flow while preventing bulk mixing of solutions. |
| Key Benefit | Prevents cross-contamination of reaction products for accurate data. |
| Common Use Cases | Controlled potential electrolysis, electrosynthesis, accurate analytical measurements. |
Need precise control for your electrochemical experiments?
An H-type cell is essential for applications where product purity and reaction accuracy are paramount. At KINTEK, we specialize in high-quality lab equipment, including electrochemical cells and consumables, designed to meet the rigorous demands of your research.
Ensure the integrity of your data and the success of your synthesis. Contact our experts today to find the perfect H-cell solution for your laboratory's needs.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry



















