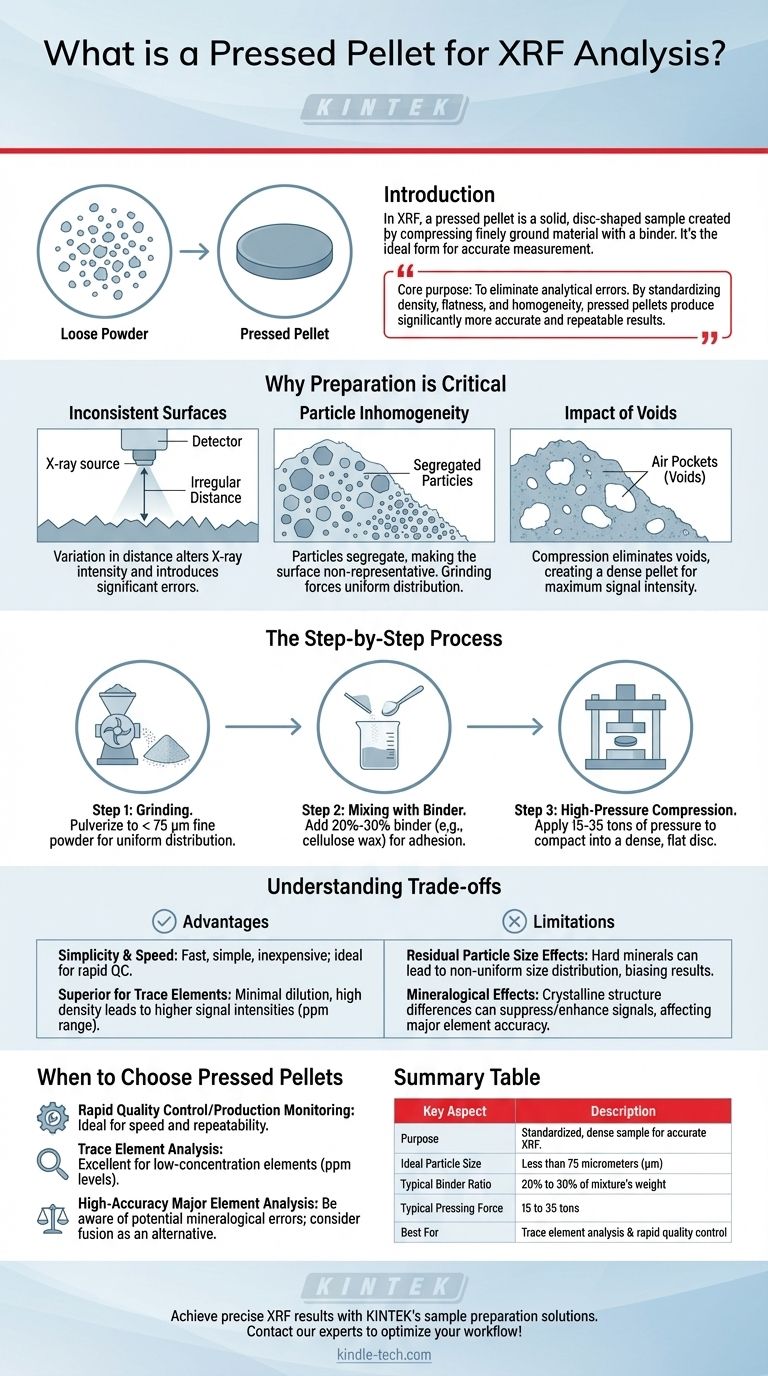
In X-ray fluorescence (XRF) analysis, a pressed pellet is a solid, disc-shaped sample created by compressing a finely ground material under high pressure. This process typically involves mixing the powdered sample with a binding agent before pressing it in a die. The goal is to transform a loose or irregular sample into a dense, homogeneous puck with a perfectly flat surface, which is the ideal form for accurate XRF measurement.
The core purpose of creating a pressed pellet is to eliminate common sources of analytical error. By standardizing the sample's density, surface flatness, and homogeneity, the pressed pellet method produces significantly more accurate and repeatable results than analyzing loose powders.

The Rationale: Why Preparation is Critical
XRF is a comparative technique, meaning it measures an unknown sample against known calibration standards. For this comparison to be valid, the sample must be presented to the instrument in a consistent and predictable way.
The Problem of Inconsistent Surfaces
An XRF spectrometer is calibrated for a precise distance between the X-ray source, the sample, and the detector. Any variation in this distance, caused by an irregular or non-flat surface, will alter the measured intensity of the X-rays and introduce significant errors into the final results.
The Issue of Particle Inhomogeneity
In a loose powder, particles can segregate based on size and density. This means the surface being analyzed may not be truly representative of the entire sample, leading to inaccurate readings. Grinding and pressing forces these particles into a fixed, uniform distribution.
The Impact of Voids
Loose powders contain air pockets, or voids, which reduce the overall density of the material being analyzed. Compression eliminates these voids, creating a dense pellet that maximizes the amount of sample material interacting with the X-ray beam and thus increases the signal intensity.
The Step-by-Step Process of Pellet Preparation
Creating a high-quality pellet is a straightforward but precise process. Each step is designed to maximize sample homogeneity and produce a durable final product.
Step 1: Grinding to a Fine Powder
The sample must first be pulverized into a very fine powder. The ideal particle size is typically less than 75 micrometers (µm). A consistent, fine grind ensures an optimal and even distribution of all components within the final pellet.
Step 2: Mixing with a Binder
The fine powder is then thoroughly mixed with a binding agent, often a cellulose wax. This binder typically makes up 20% to 30% of the mixture's weight. It serves to help the sample particles adhere to one another during compression, resulting in a stable, mechanically sound pellet.
Step 3: High-Pressure Compression
The powder-binder mixture is poured into a die, which is then placed in a laboratory press. The mixture is subjected to immense pressure, usually between 15 and 35 tons. This force compacts the powder, expels trapped air, and forms the dense, solid pellet with a smooth, flat analytical surface.
Understanding the Trade-offs of Pressed Pellets
While powerful, the pressed pellet method is not without its limitations. Understanding its advantages and disadvantages is key to using it appropriately.
Advantage: Simplicity and Speed
Compared to more complex methods like fusion, preparing pressed pellets is relatively fast, simple, and inexpensive. It requires only a pulverizing mill and a press, making it highly suitable for production control environments where rapid turnaround is necessary.
Advantage: Superior for Trace Elements
Because the process involves minimal dilution (only the binder is added) and creates high sample density, it leads to higher signal intensities. This makes pressed pellets an excellent choice for analyzing elements present in the parts-per-million (ppm) range.
Limitation: Residual Particle Size Effects
While grinding helps, it cannot completely eliminate particle size effects. Some minerals are harder to grind than others, which can lead to a non-uniform distribution of particle sizes. This can subtly bias the results, particularly for major elements.
Limitation: Mineralogical Effects
The crystalline structure and chemical matrix of different minerals can influence how they respond to X-rays. Pressing a sample does not alter its mineralogy. These effects can suppress or enhance the signal from certain elements, impacting the accuracy of major element quantification.
When to Choose Pressed Pellets for Your Analysis
Use the following guidelines to decide if this method aligns with your analytical goals.
- If your primary focus is rapid quality control or production monitoring: Pressed pellets are ideal due to their speed of preparation and high repeatability, especially when working with narrow calibration ranges.
- If your primary focus is analyzing trace elements (ppm levels): This method is excellent because the high sample density maximizes signal intensity for low-concentration elements.
- If your primary focus is high-accuracy analysis of major elements: Be aware of the potential for errors from mineralogical effects and consider alternative methods like fusion, which eliminates these issues.
Ultimately, mastering the pressed pellet technique provides a powerful, reliable, and cost-effective tool for a wide range of analytical challenges.
Summary Table:
| Key Aspect | Description |
|---|---|
| Purpose | To create a standardized, dense sample for accurate XRF measurement. |
| Ideal Particle Size | Less than 75 micrometers (µm). |
| Typical Binder Ratio | 20% to 30% of the mixture's weight. |
| Typical Pressing Force | 15 to 35 tons. |
| Best For | Trace element analysis and rapid quality control. |
Achieve precise and reliable XRF results with KINTEK's sample preparation solutions.
Proper sample preparation is the foundation of accurate analysis. Our range of high-quality laboratory presses, dies, and consumables are designed to help you create perfect pressed pellets every time, ensuring your XRF data is both accurate and repeatable.
Whether you're focused on quality control or trace element detection, KINTEK has the equipment and expertise to support your laboratory's specific needs.
Contact our experts today to discuss how we can optimize your XRF sample preparation workflow!
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is the pellet technique in IR? Master Solid Sample Preparation for Clear Spectroscopy
- Why use KBr for IR? Achieve Clear, Unobstructed Spectra for Solid Samples
- What is the advantage of KBr? Unmatched IR Transparency for Precise Spectroscopy
- Are hydraulic presses powered by water? Discover the critical role of hydraulic oil.
- Why are KBr pellets used in FTIR? Achieve Clear, Accurate Solid Sample Analysis



















