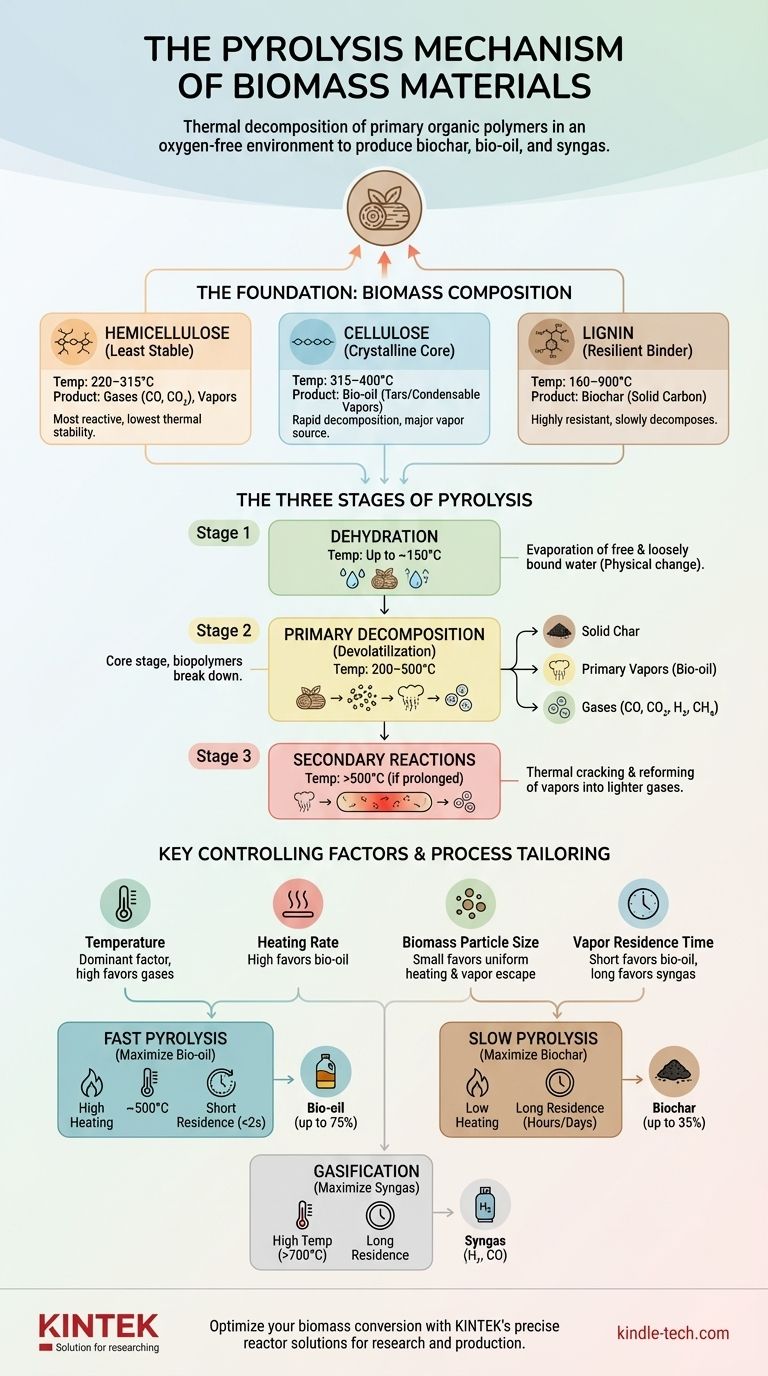
At its core, the pyrolysis mechanism of biomass is the thermal decomposition of its primary organic polymers in an oxygen-free environment. It is not a single chemical reaction but a complex, multi-stage process where cellulose, hemicellulose, and lignin break down at different temperatures to produce a mixture of solid (biochar), liquid (bio-oil), and gaseous (syngas) products.
The key to understanding biomass pyrolysis is to see it as a controlled disassembly process. The final output is not accidental; it is a direct consequence of which components of the biomass break down, when they break down, and what happens to the resulting vapors before they exit the reactor.

The Foundation: Biomass Composition
To grasp the mechanism, you must first understand the three main building blocks of biomass. Each component decomposes differently, acting as a distinct input to the overall process.
Hemicellulose: The Least Stable Component
Hemicellulose is a branched polymer that is the most reactive and least thermally stable of the three. It begins to decompose at the lowest temperature range, typically 220–315°C. Its decomposition yields a mix of volatile gases (CO, CO2) and condensable organic vapors, but contributes less to biochar formation.
Cellulose: The Crystalline Core
Cellulose is a long, linear, and crystalline polymer that is more stable than hemicellulose. It decomposes rapidly over a narrower and higher temperature range, generally 315–400°C. This rapid breakdown is responsible for producing the majority of the condensable vapors (tars) that form bio-oil upon cooling.
Lignin: The Resilient Binder
Lignin is a complex, aromatic polymer that acts as the structural glue in biomass. It is highly resistant to thermal degradation, decomposing very slowly over a wide temperature range (160–900°C). Lignin is the primary source of biochar, as its stable aromatic rings tend to rearrange and condense into a solid carbon structure rather than breaking into volatile fragments.
The Three Stages of the Pyrolysis Reaction
The overall mechanism unfolds in a sequence of overlapping physical and chemical stages as the temperature of the biomass particle increases.
Stage 1: Dehydration
At temperatures up to around 150°C, the primary process is the evaporation of free and loosely bound water from the biomass. This is a physical change, not a chemical decomposition, but it is a critical energy-consuming step that must occur before pyrolysis can begin.
Stage 2: Primary Decomposition (Devolatilization)
This is the heart of the pyrolysis process, occurring between roughly 200°C and 500°C. During this stage, the three biopolymers break down into a mix of primary products:
- Solid Char: A carbon-rich residue formed from the condensation of lignin and other non-volatile components.
- Primary Vapors: A complex aerosol of condensable organic molecules (which form bio-oil).
- Gases: Non-condensable "permanent" gases like CO, CO₂, H₂, and CH₄.
The relative proportion of these products is determined by the biomass composition and the heating conditions.
Stage 3: Secondary Reactions
As the primary vapors and gases are released, they travel through the hot reactor. If the temperature is high enough (typically >500°C) and they remain in the hot zone long enough, they undergo secondary reactions. These include thermal cracking, repolymerization, and reforming, which break down larger vapor molecules into smaller, lighter gases and can also form secondary char on surfaces.
Key Factors That Control the Mechanism
The final product yields are not fixed. They are directly controlled by the process conditions, which influence which reaction pathways are favored.
Temperature and Heating Rate
Temperature is the most dominant factor. Higher temperatures favor the cracking of vapors into permanent gases. Heating rate dictates how quickly the biomass particle reaches the target temperature. A high heating rate causes rapid decomposition that favors the formation and escape of vapors, maximizing liquid yield.
Biomass Composition and Particle Size
The inherent ratio of cellulose, hemicellulose, and lignin pre-determines the potential yields. Particle size is critical because smaller particles heat up more quickly and uniformly, and the volatile products have a shorter distance to travel to escape, minimizing the chance for secondary reactions.
Vapor Residence Time
This is the amount of time the hot vapors and gases spend inside the reactor. A short residence time is essential for preserving the primary vapors to maximize bio-oil yield. A long residence time allows for extensive secondary cracking, which maximizes syngas production at the expense of oil.
Understanding the Trade-offs: Fast vs. Slow Pyrolysis
The interplay of these factors leads to two primary modes of operation, each designed to maximize a different product.
Fast Pyrolysis: Maximizing Bio-oil
This process uses very high heating rates, moderate temperatures (~500°C), and a short vapor residence time (<2 seconds). The goal is to rapidly break down the cellulose and hemicellulose and immediately remove the vapors before they can undergo secondary reactions, thereby maximizing the yield of liquid bio-oil (up to 75% by weight).
Slow Pyrolysis: Maximizing Biochar
Also known as carbonization, this process uses low heating rates and a much longer residence time (hours to days). These conditions favor the gradual removal of volatiles and promote the rearrangement and condensation reactions that form a stable, carbon-rich biochar (up to 35% by weight).
Tailoring the Mechanism to Your Goal
By understanding the governing principles, you can manipulate the pyrolysis mechanism to achieve a specific outcome.
- If your primary focus is producing liquid biofuel (bio-oil): Employ fast pyrolysis with high heating rates, moderate temperatures (~500°C), and small biomass particles to ensure rapid vapor escape.
- If your primary focus is creating stable biochar for soil or filtration: Use slow pyrolysis with low heating rates and long processing times to maximize solid yield and carbon stability.
- If your primary focus is generating syngas for energy: Use high temperatures (>700°C) and longer vapor residence times to intentionally promote the secondary cracking of all volatile compounds into simple gases like H₂ and CO.
Mastering the pyrolysis mechanism transforms it from a simple heating process into a precise engineering tool for converting biomass into valuable, tailored products.
Summary Table:
| Component | Decomposition Temp. | Primary Product |
|---|---|---|
| Hemicellulose | 220–315°C | Gases (CO, CO₂), Vapors |
| Cellulose | 315–400°C | Bio-oil (Condensable Vapors) |
| Lignin | 160–900°C | Biochar (Solid Carbon) |
| Process Type | Key Conditions | Target Product |
| Fast Pyrolysis | High heating rate, ~500°C, short vapor residence time | Maximize Bio-oil (up to 75%) |
| Slow Pyrolysis | Low heating rate, long residence time | Maximize Biochar (up to 35%) |
| Gasification | High temperature (>700°C), long vapor residence time | Maximize Syngas (H₂, CO) |
Ready to optimize your biomass conversion process? Whether your goal is to maximize bio-oil for fuel, produce stable biochar for soil enhancement, or generate syngas for energy, KINTEK has the expertise and reliable lab equipment to help you master the pyrolysis mechanism. Our reactors and consumables are designed for precise control over temperature, heating rate, and residence time—the critical factors that determine your final product yields. Contact our experts today to discuss how we can tailor a solution for your specific biomass research or production needs.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Test Sieves and Sieving Machines
- Vacuum Dental Porcelain Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















