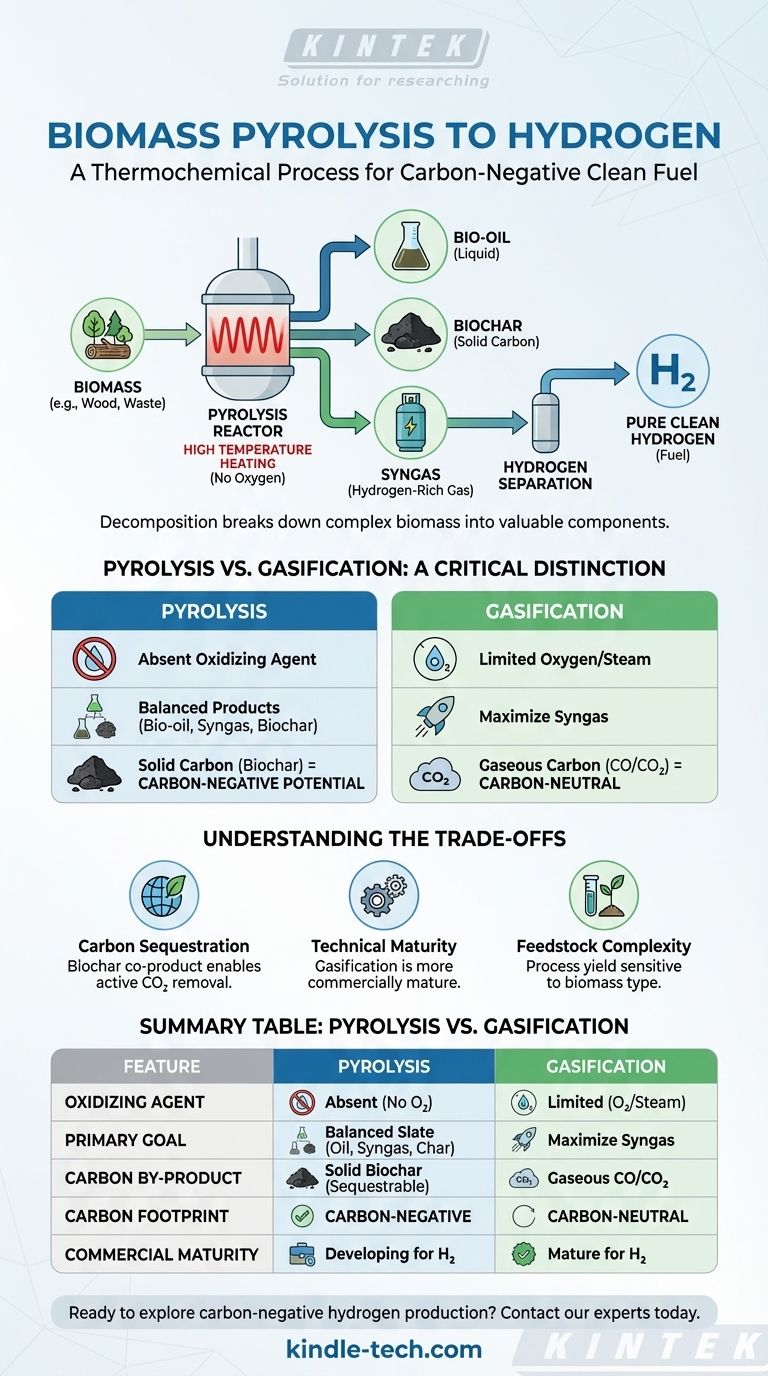
In essence, the pyrolysis of biomass is a thermochemical process that heats organic material, like wood or agricultural waste, to high temperatures in an environment completely devoid of oxygen. This process breaks down the complex materials into a hydrogen-rich gas (syngas), a liquid (bio-oil), and a solid, carbon-rich charcoal (biochar). The hydrogen is then separated from the syngas for use as a clean fuel.
The critical difference between pyrolysis and other methods is its potential for carbon-negative hydrogen production. By converting much of the biomass carbon into a stable, solid biochar instead of gaseous CO₂, the process creates a direct path for carbon sequestration.

How Biomass Pyrolysis Creates Hydrogen
Biomass pyrolysis is fundamentally about thermal decomposition. Instead of burning the material, it is intensely heated, causing its chemical structure to break apart into simpler, more valuable components.
The Core Principle: Heating Without Oxygen
The defining feature of pyrolysis is the absence of an oxidizing agent like oxygen or steam during the primary heating stage. This prevents combustion and ensures the biomass decomposes into the three primary products.
This process is distinct from combustion (burning with ample oxygen) and gasification (reacting with a limited amount of oxygen or steam).
The Three Key Products
The decomposition of biomass yields a mix of outputs that can all be further processed to maximize hydrogen yield.
- Syngas: A mixture of gases including hydrogen, carbon monoxide, carbon dioxide, and methane. This is the most direct source of hydrogen from the process.
- Bio-oil: A liquid product that can be upgraded through a secondary process like steam reforming to produce additional hydrogen.
- Biochar: A stable, solid charcoal. This product is key to the technology's environmental benefit, as it locks carbon away in a form that can be added to soil or sequestered.
Extracting the Hydrogen
Once the initial pyrolysis step is complete, hydrogen is separated from the syngas, typically using specialized membranes or pressure swing adsorption (PSA) systems. Further reactions, like the water-gas shift reaction, can also be used to convert the carbon monoxide in the syngas into additional hydrogen.
Pyrolysis vs. Gasification: A Critical Distinction
While both are thermochemical processes for producing hydrogen from biomass, their methods and primary goals differ significantly. Understanding this is key to evaluating their respective roles.
The Role of an Oxidizing Agent
Pyrolysis is defined by the absence of oxygen. It is pure thermal decomposition.
Gasification is a mature technology that uses a controlled, limited amount of an oxidizing agent (like oxygen, air, or steam) at high temperatures (>700°C) to intentionally convert biomass primarily into syngas.
Primary Outputs and Goals
The goal of gasification is to maximize the production of syngas for conversion into hydrogen. It is designed to turn as much of the solid biomass as possible into a gaseous fuel.
The goal of pyrolysis is to produce a balanced slate of bio-oil, biochar, and syngas. Different process conditions (e.g., fast vs. slow pyrolysis) can be used to favor one product over the others.
The Carbon By-product: Solid vs. Gaseous
This is the most important difference from a climate perspective.
In gasification, most of the carbon from the biomass exits the process as gaseous carbon monoxide and carbon dioxide.
In pyrolysis, a significant portion of the carbon is captured in the solid biochar. This creates a tangible product that can be permanently sequestered, offering a path to carbon-negative hydrogen.
Understanding the Trade-offs
While promising, biomass pyrolysis for hydrogen production is not yet as established as other methods and comes with its own set of considerations.
The Promise of Carbon Sequestration
The unique advantage of pyrolysis is its biochar co-product. If this solid carbon is sequestered or used in applications like soil amendment, the entire process can have a negative carbon footprint, actively removing CO₂ from the atmosphere.
Technical and Commercial Maturity
Biomass gasification is a more mature, commercially demonstrated technology for large-scale syngas and hydrogen production.
Pyrolysis of biomass is well-established for producing bio-oil and biochar, but its integration and optimization specifically for large-scale hydrogen production are less commercialized compared to gasification or steam reforming of natural gas.
Feedstock and Process Complexity
The yield and composition of the three products (bio-oil, syngas, biochar) are highly sensitive to the type of biomass used and the specific pyrolysis conditions (temperature, heating rate). This requires precise control and optimization, adding a layer of complexity to the operation.
Making the Right Choice for Your Goal
Selecting the appropriate technology depends entirely on your strategic priorities, whether they are focused on technological readiness, environmental impact, or economic viability.
- If your primary focus is leveraging mature, proven technology: Biomass gasification is the more established and commercially available pathway for converting solid biomass into hydrogen at scale.
- If your primary focus is maximizing carbon capture and achieving a carbon-negative footprint: Pyrolysis offers a unique and powerful advantage by converting biomass carbon into stable, solid biochar that can be sequestered.
- If your primary focus is producing a slate of valuable co-products: Pyrolysis provides flexibility to produce bio-oil and biochar alongside hydrogen, creating multiple potential revenue streams.
Ultimately, pyrolysis represents a promising frontier in green hydrogen, offering a unique mechanism for simultaneous energy production and carbon removal.
Summary Table:
| Feature | Pyrolysis | Gasification |
|---|---|---|
| Oxidizing Agent | Absent | Limited oxygen/steam |
| Primary Goal | Balanced slate of bio-oil, syngas, and biochar | Maximize syngas production |
| Carbon By-product | Solid biochar (sequestrable) | Gaseous CO/CO₂ |
| Carbon Footprint Potential | Carbon-negative | Carbon-neutral |
| Commercial Maturity | Developing for H₂ production | Mature for H₂ production |
Ready to explore carbon-negative hydrogen production for your operations?
KINTEK specializes in advanced laboratory equipment and consumables for renewable energy research and development. Whether you are developing pyrolysis processes, analyzing bio-oil, or characterizing biochar, our precision tools can support your innovation in green hydrogen.
Contact our experts today to discuss how our solutions can accelerate your biomass-to-hydrogen projects.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- What is the temperature and residence time for pyrolysis? Master the Parameters for Biochar, Bio-Oil, or Syngas
- What are the steps of pyrolysis? A Complete Guide to the 3-Phase Process
- How does biomass break down during pyrolysis? A Guide to Controlled Thermal Decomposition
- What are the disadvantages of a rotary kiln? High Costs and Operational Challenges
- What is the function of a pyrolysis reactor? Transform Waste into Valuable Resources with Thermal Cracking
- What does the efficiency of the pyrolysis process depend on? Optimize Feedstock & Reactor Control
- What are the main products formed from the pyrolysis process? A Guide to Bio-char, Bio-oil, and Syngas
- What happens to plastic after pyrolysis? Discover How to Turn Waste into Fuel and Chemicals



















