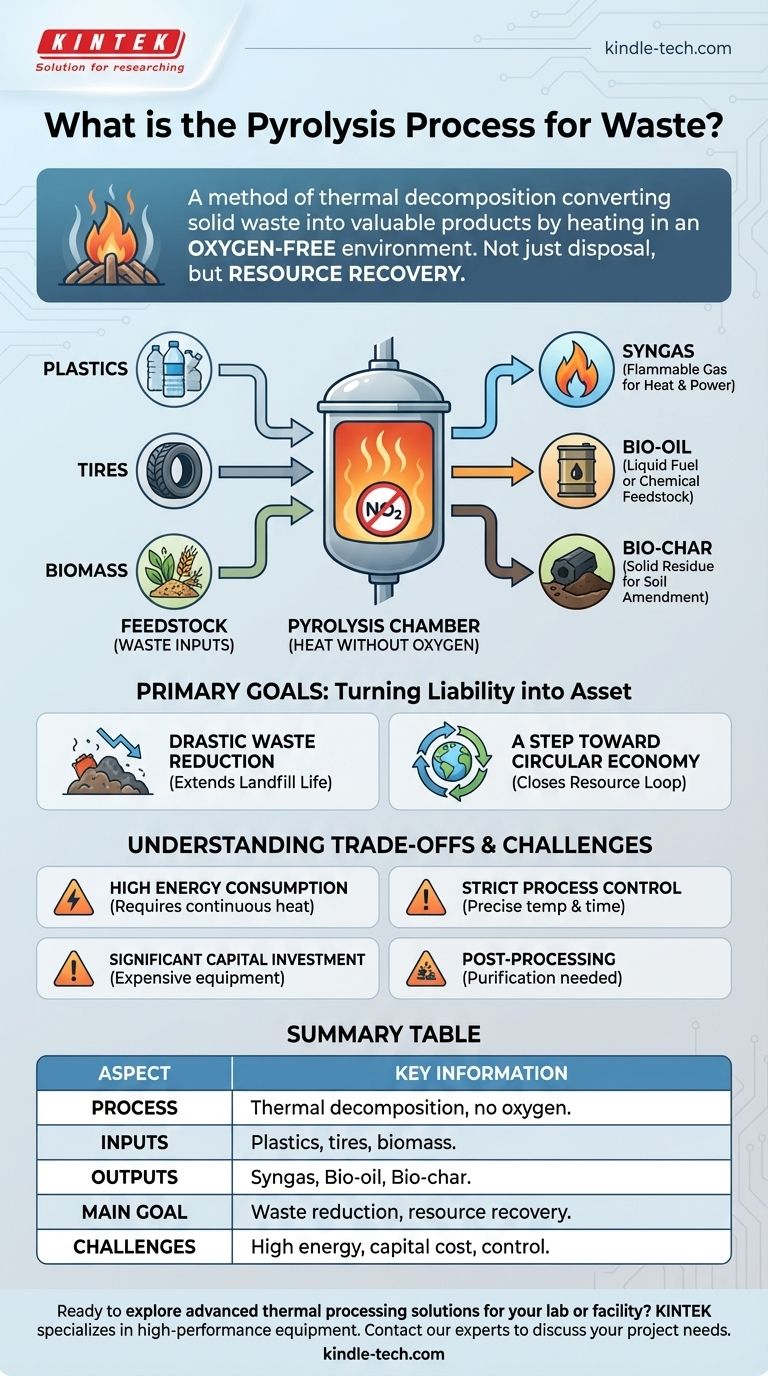
Pyrolysis is a method of thermal decomposition used to convert solid waste into valuable products. It involves heating materials like plastics, tires, or biomass to high temperatures in an environment completely devoid of oxygen, which prevents the material from simply burning and instead breaks it down into useful components: syngas, bio-oil, and bio-char.
At its core, pyrolysis is not just a waste disposal technique but a resource recovery technology. It transforms materials otherwise destined for a landfill into potential fuels and industrial products, though this conversion comes with significant energy and capital costs.

How Pyrolysis Transforms Waste
Pyrolysis is a carefully controlled thermochemical process. Understanding its core mechanism reveals why it's a promising, albeit complex, solution for waste management.
The Critical Role of Heat Without Oxygen
The defining characteristic of pyrolysis is the absence of oxygen. When waste is heated, the intense thermal energy breaks the chemical bonds within the material.
Without oxygen, combustion (burning) cannot occur. Instead of turning into ash and smoke, the complex organic materials decompose into simpler, more valuable molecules.
Common Inputs (Feedstock)
Pyrolysis is versatile and can process various types of organic and carbon-based solid waste. Common feedstocks include post-consumer plastics, used vehicle tires, and organic biomass such as agricultural waste.
The Three Valuable Outputs
The process consistently yields three primary products, each with distinct uses.
- Syngas: A mixture of flammable gases, primarily hydrogen and carbon monoxide. This can be burned directly to generate heat and electricity.
- Bio-oil: A liquid, also known as pyrolysis oil, that can be refined and used as a fuel or as a feedstock for producing other chemicals.
- Bio-char: A stable, solid residue rich in carbon. It can be used as a soil amendment to improve fertility or as a raw material for producing activated carbon.
The Primary Goal: Turning Liability into an Asset
The fundamental driver for adopting pyrolysis is its ability to reframe the concept of waste. It treats discarded materials as an untapped resource, offering significant environmental and economic benefits.
Drastic Waste Volume Reduction
Pyrolysis dramatically reduces the sheer volume of waste that must be sent to landfills. This extends the life of existing landfills and minimizes the land required for waste disposal, a critical issue for densely populated areas.
A Step Toward a Circular Economy
By converting waste into usable fuels and materials, pyrolysis helps to close the resource loop. It recovers value from discarded products, reducing the need to extract new virgin resources and lowering the overall environmental impact of manufacturing and energy production.
Understanding the Trade-offs and Challenges
While promising, pyrolysis is not a perfect solution. A clear-eyed assessment of its challenges is necessary to understand its practical applications and limitations.
High Energy Consumption
The process is fundamentally energy-intensive. It requires a significant and continuous input of heat to maintain the high temperatures needed for efficient thermal decomposition. This energy cost must be factored into any economic or environmental analysis.
The Need for Strict Process Control
Pyrolysis is not a simple "set it and forget it" operation. The efficiency of the process and the quality of the end products depend heavily on maintaining specific conditions, particularly temperature and processing time.
Significant Capital Investment
Building a pyrolysis facility involves high upfront capital costs. The specialized reactors and equipment required for high-temperature, oxygen-free processing are expensive to manufacture and install.
Post-Processing and Purification
The outputs of pyrolysis are rarely pure enough for immediate use. The syngas, bio-oil, and bio-char often require separation and purification steps to remove contaminants before they can be sold or used effectively, adding complexity and cost to the operation.
Making the Right Choice for Your Goal
Pyrolysis is a powerful tool, but its suitability depends entirely on the primary objective.
- If your primary focus is large-scale waste diversion: Pyrolysis is an extremely effective technology for reducing landfill dependence and recovering value from materials like plastics and tires.
- If your primary focus is low-cost energy generation: The high capital and energy inputs mean that the economic viability must be carefully assessed against the local cost of waste disposal and the market value of the energy products.
- If your primary focus is environmental sustainability: Pyrolysis offers a clear advantage over landfilling, but a complete lifecycle assessment is needed to ensure the energy consumed by the process doesn't outweigh the benefits.
Ultimately, pyrolysis represents a sophisticated and promising pathway for managing waste, provided its operational challenges are met with careful planning and investment.
Summary Table:
| Aspect | Key Information |
|---|---|
| Process | Thermal decomposition of waste in the absence of oxygen. |
| Common Inputs | Plastics, tires, biomass (agricultural waste). |
| Primary Outputs | Syngas (fuel), Bio-oil (fuel/chemicals), Bio-char (soil amendment). |
| Main Goal | Waste volume reduction and resource recovery for a circular economy. |
| Key Challenges | High energy consumption, significant capital investment, need for strict process control. |
Ready to explore advanced thermal processing solutions for your laboratory or facility? KINTEK specializes in high-performance lab equipment and consumables. Whether you're researching pyrolysis processes or scaling up waste-to-energy technology, our expertise can help you achieve precise temperature control and reliable results. Contact our experts today to discuss how we can support your specific project needs and help you turn waste into a valuable resource.
Visual Guide
Related Products
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- How is the operational mode of bed motion selected for a rotary kiln? Optimize Heat Transfer and Material Homogeneity
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker
- What are the equipment for pyrolysis laboratory? Choosing the Right Reactor for Your Research
- What is the meaning of rotary furnace? Achieve Superior Uniformity in Continuous Heat Treatment
- What is a rotary kiln reactor? A Guide to Industrial Thermal Processing



















