In X-ray Fluorescence (XRF), qualitative analysis is the process of identifying which elements are present in a sample. It works by measuring the unique energy signatures of the fluorescent X-rays that each element emits when excited. This allows you to determine the elemental composition of a material without needing to know the specific concentration of each element.
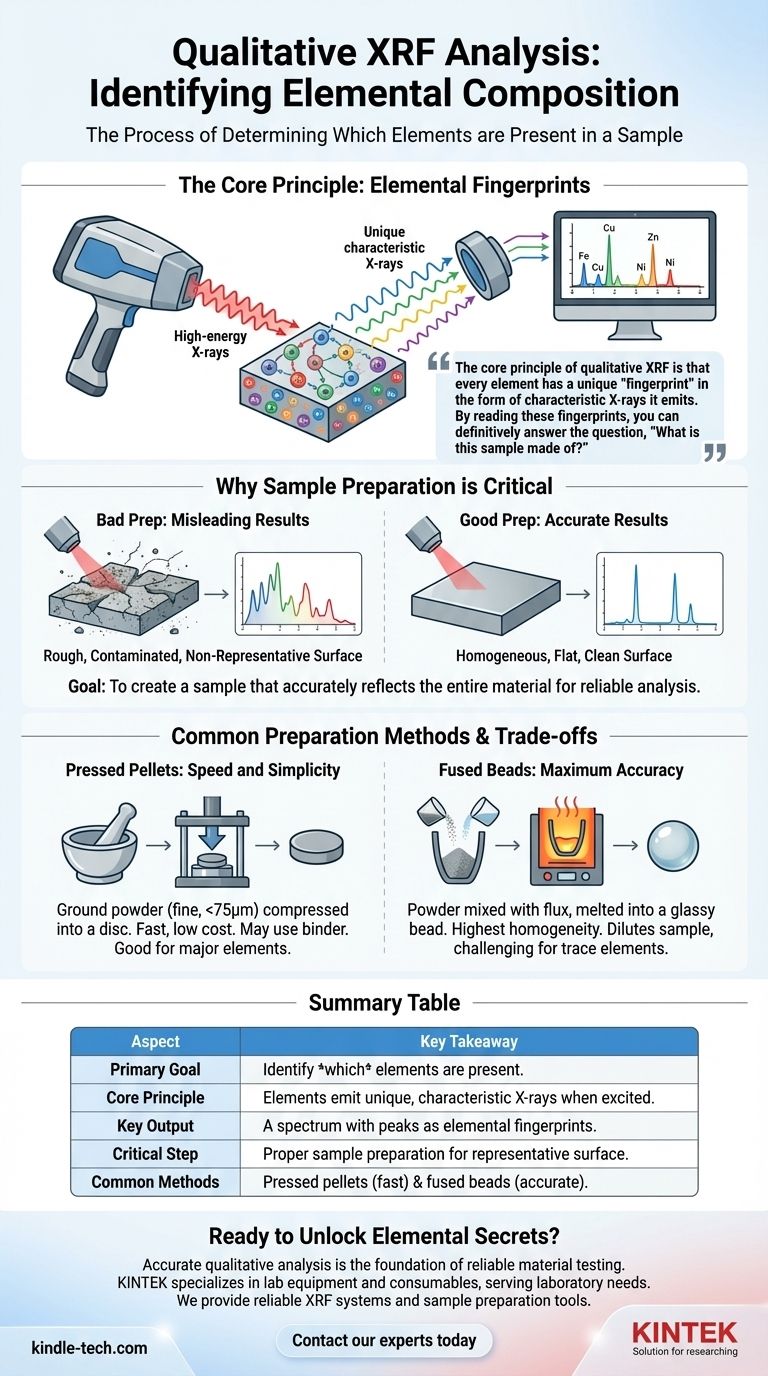
The core principle of qualitative XRF is that every element has a unique "fingerprint" in the form of characteristic X-rays it emits. By reading these fingerprints, you can definitively answer the question, "What is this sample made of?"

The Core Principle: Elemental Fingerprints
Qualitative analysis is the foundational step in XRF. It provides the essential inventory of elements upon which any further quantitative analysis (measuring how much of each element is present) is built.
How XRF Generates a Signal
The XRF instrument bombards a sample with high-energy X-rays. This energy is absorbed by the atoms within the sample, momentarily exciting them into an unstable state.
Characteristic X-ray Emission
To return to a stable state, the atoms in the sample release this excess energy by emitting their own fluorescent X-rays. Crucially, the energy or wavelength of these emitted X-rays is unique and predictable for each specific element.
Reading the Elemental Spectrum
The instrument's detector measures the energy of all the X-rays coming from the sample. The result is a spectrum with peaks at different energy levels. Each peak acts as a definitive marker, corresponding directly to a specific element present in the material.
Why Sample Preparation is Critical
The XRF beam only analyzes the surface of the sample. If the surface is not representative of the whole material, your results will be misleading. Proper preparation ensures the analysis is accurate and reliable.
The Goal: Homogeneity and Representation
The fundamental objective is to create a sample that is homogeneous, meaning its composition is uniform throughout. This ensures that the small area being analyzed accurately reflects the entire sample.
For Solid Samples: A Clean, Flat Surface
For solid materials like metals or plastics, the primary requirement is a flat, smooth, and clean surface. Any roughness or contamination can interfere with the X-ray measurement.
Preparation often involves polishing the sample to achieve a smooth finish. It is critical to clean the surface with a file or other tool, using separate tools for different sample types to avoid cross-contamination.
Common Preparation Methods and Their Trade-offs
For materials that are not already in a solid, flat form (like powders, soils, or irregular fragments), they must be processed into a suitable state.
Pressed Pellets: Speed and Simplicity
This is a very common method due to its speed and low cost. The sample is first ground into a fine powder (typically smaller than 75 micrometers) and then compressed under high pressure using a die set to form a solid, disc-shaped pellet.
If the powder does not bind well on its own, a wax binder can be mixed in to help it cohere.
Fused Beads: Maximum Accuracy
For the highest level of homogeneity, a fused bead is created. This involves mixing the powdered sample with a flux agent (like a lithium borate salt) and heating it at high temperatures until it melts into a glassy, disc-like bead.
While this creates an almost perfectly homogeneous sample, it also dilutes the original material. This can make it more difficult to detect elements present in very low concentrations (trace elements).
Making the Right Choice for Your Goal
Your analytical goal dictates the necessary level of sample preparation.
- If your primary focus is rapid identification of major elements: A clean surface on a solid sample or a simple pressed pellet is often sufficient.
- If your primary focus is the most accurate and repeatable identification: Creating a fused bead eliminates issues related to particle size and mineral composition, providing the most reliable data.
- If your primary focus is avoiding contamination: Meticulous cleaning of solid surfaces and dedicated grinding/pressing equipment is non-negotiable.
Ultimately, successful qualitative analysis hinges on preparing a sample that accurately represents the material you need to understand.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Primary Goal | To identify which elements are present in a sample. |
| Core Principle | Each element emits unique, characteristic X-rays when excited. |
| Key Output | A spectrum with peaks that act as elemental fingerprints. |
| Critical Step | Proper sample preparation to ensure a representative, homogeneous surface. |
| Common Methods | Pressed pellets (fast, cost-effective) and fused beads (highly accurate). |
Ready to Unlock the Elemental Secrets of Your Materials?
Accurate qualitative analysis is the foundation of reliable material testing. The right equipment and expertise are essential for generating definitive results.
KINTEK specializes in lab equipment and consumables, serving laboratory needs. We provide the reliable XRF systems and sample preparation tools—like presses and fusion furnaces—you need to ensure your qualitative analysis is a success.
Let us help you achieve precise and dependable elemental identification.
Contact our experts today to discuss your application and find the perfect solution for your lab.
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Vacuum Cold Trap Chiller Indirect Cold Trap Chiller
People Also Ask
- How are laboratory hydraulic presses and pellet dies used in mechanochemistry? Unlock Precision in Solid-State Research
- What are the hazards of a hydraulic press machine? Beyond Crushing, Uncover Systemic Risks
- What is a pressed pellet for XRF analysis? A Guide to Accurate Sample Preparation
- What role does a laboratory hydraulic press play in preparing LixScCl3+x samples for EIS? Achieve Reliable Conductivity
- What is the primary function of a powder pellet press in the preparation of fillers? Achieve Superior Ceramic Joining
- How many psi is a hydraulic press force? Understand Pressure vs. Force for Your Application
- How do you prepare a KBr pellet for IR spectroscopy? Master the Key Steps for a Clear Spectrum
- What are the advantages of pressing and sintering? Achieve Complex, High-Strength Parts Cost-Effectively










