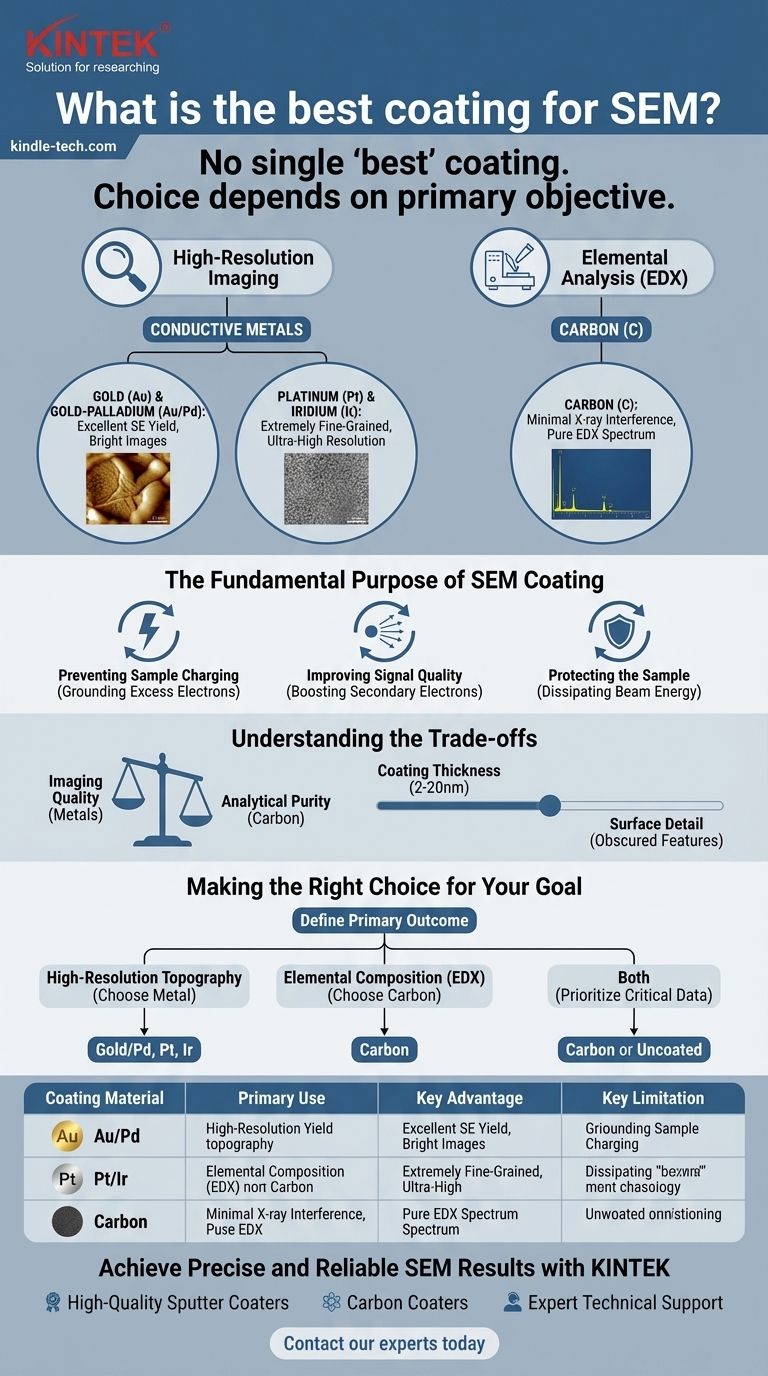
There is no single 'best' coating for SEM analysis. The ideal choice is entirely dependent on your primary objective. For generating high-resolution images of a sample's surface, a conductive metal like gold or platinum is the standard. However, if your goal is to determine the sample's elemental composition using techniques like EDX, carbon is the only appropriate choice.
The selection of an SEM coating is a critical analytical decision, not a simple preparation step. Your choice determines the outcome: metals are chosen for maximizing image quality, while carbon is used to preserve the accuracy of elemental analysis.

The Fundamental Purpose of an SEM Coating
Before choosing a material, it is essential to understand why coating is necessary for many samples. The electron beam used in a scanning electron microscope requires the sample to be conductive to function correctly.
Preventing Sample Charging
Non-conductive materials accumulate electrons from the beam on their surface. This phenomenon, known as charging, creates bright spots, image distortion, and other artifacts that make the resulting image unusable. A thin conductive coating provides a path for these excess electrons to travel to the ground, eliminating the issue.
Improving Signal Quality
The interaction of the electron beam with the sample generates several signals, but the most common for imaging are secondary electrons (SE). Heavy metals like gold and platinum are excellent emitters of secondary electrons. Coating a sample with one of these materials significantly boosts the SE signal, leading to a much better signal-to-noise ratio and a clearer, more detailed image of the surface topography.
Protecting the Sample
For delicate biological or polymeric specimens, the intense electron beam can cause damage. A conductive coating helps to dissipate the energy and heat from the beam more effectively, offering a degree of protection to beam-sensitive samples.
A Guide to Common Coating Materials
While many materials can be used, the choice almost always comes down to a few industry standards, each suited for a specific application.
Gold (Au) and Gold-Palladium (Au/Pd)
These are the most common coatings for general-purpose, high-quality imaging. Gold is highly conductive and has a high secondary electron yield, producing bright, clear images. Adding palladium creates a slightly finer grain structure, which can be beneficial for imaging at higher magnifications.
Platinum (Pt) and Iridium (Ir)
When extremely high magnification is required, the grain size of the coating itself can become a limiting factor. Platinum and iridium produce an exceptionally fine-grained coating, making them the preferred choice for ultra-high-resolution imaging where the subtle texture of the coating will not obscure nanoscale surface features.
Carbon (C)
Carbon is the definitive choice for any analysis involving Energy-Dispersive X-ray Spectroscopy (EDX or EDS). Because carbon has a very low atomic number, its characteristic X-ray peak is low-energy and does not interfere with the peaks of other elements you are trying to detect. Using a metal coating like gold would add strong, unwanted peaks to your spectrum, corrupting your elemental analysis.
Understanding the Trade-offs
Choosing a coating material is an exercise in managing competing priorities. The ideal material for one type of analysis is often the worst for another.
Imaging Quality vs. Analytical Purity
This is the primary trade-off. The heavy metals that produce the best images (Au, Pt) will contaminate your EDX spectrum. The carbon that ensures a clean EDX spectrum provides a much lower signal yield for imaging, resulting in images that are often less sharp and have more noise compared to those from a gold-coated sample.
Coating Thickness vs. Surface Detail
A coating must be thick enough to ensure full conductivity across the sample surface. However, a coating that is too thick will obscure the very features you intend to observe. A typical coating is only 2-20 nanometers thick—a delicate balance between preventing charging and preserving the original surface morphology.
Material Interaction
The chosen coating material must adhere well to the sample without reacting with it or altering its structure. The sputtering process itself can heat the sample, which may be a concern for highly sensitive materials.
Making the Right Choice for Your Goal
To select the correct coating, you must first define your most important expected outcome.
- If your primary focus is high-resolution imaging of surface topography: Choose a fine-grained, conductive metal. Gold-palladium is excellent for general use, while platinum or iridium is superior for ultra-high-resolution work.
- If your primary focus is determining the elemental composition (EDX/EDS): You must use a carbon coating to avoid signal interference and ensure the analytical purity of your results.
- If you need to perform both imaging and EDX on the same sample: Prioritize the most critical data. This often means using a carbon coat and accepting a lower-quality image, or performing an initial analysis on an uncoated portion of the sample if it is stable enough under the beam.
Ultimately, selecting the right coating transforms it from a simple preparation step into a powerful tool for achieving precise, reliable results.
Summary Table:
| Coating Material | Primary Use | Key Advantage | Key Limitation |
|---|---|---|---|
| Gold (Au) / Gold-Palladium (Au/Pd) | High-resolution imaging | Excellent conductivity & high secondary electron yield | Interferes with EDX analysis |
| Platinum (Pt) / Iridium (Ir) | Ultra-high-resolution imaging | Extremely fine-grained coating | Interferes with EDX analysis |
| Carbon (C) | Elemental Analysis (EDX/EDS) | Minimal interference with X-ray spectra | Lower signal yield for imaging |
Achieve Precise and Reliable SEM Results with KINTEK
Choosing the correct coating is a critical step that directly impacts the quality of your SEM data. The expert team at KINTEK understands these nuances and is here to help you select the ideal coating material and equipment for your specific application—whether your priority is stunning high-resolution images or analytically pure elemental composition data.
We provide the solutions your laboratory needs to succeed:
- High-Quality Sputter Coaters for consistent, fine-grained metal coatings.
- Carbon Coaters designed for contamination-free EDX analysis.
- Expert Technical Support to guide you in sample preparation and method development.
Let KINTEK, your partner in laboratory excellence, empower your research.
Contact our experts today to discuss your SEM coating requirements and ensure your samples are prepared for success.
Visual Guide
Related Products
- Custom CVD Diamond Coating for Lab Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Isostatic Molding Pressing Molds for Lab
People Also Ask
- What is the required temperature for ash content determination? Achieve Accurate Mineral Analysis in Your Lab
- What is the high temperature of quartz? Key Thresholds for Crystalline vs. Fused Silica
- What is the process of sintering in chemistry? A Step-by-Step Guide to Solid-State Fusion
- What safety feature do most ULT freezers have to protect stored samples? Redundancy and Alarm Systems
- What protective roles do Sealing Gaskets and Support Grids play in oil-water separation? Ensure High-Pressure Integrity
- How are polymers used in the sintering process? Master Porosity and Strength with Expert Techniques
- What is the temperature range for pyrolysis? Optimize for Biochar, Bio-oil, or Syngas
- Why is a high-precision co-precipitation apparatus required for Mg-Al-Zn synthesis? Optimize Adsorbent Performance.










