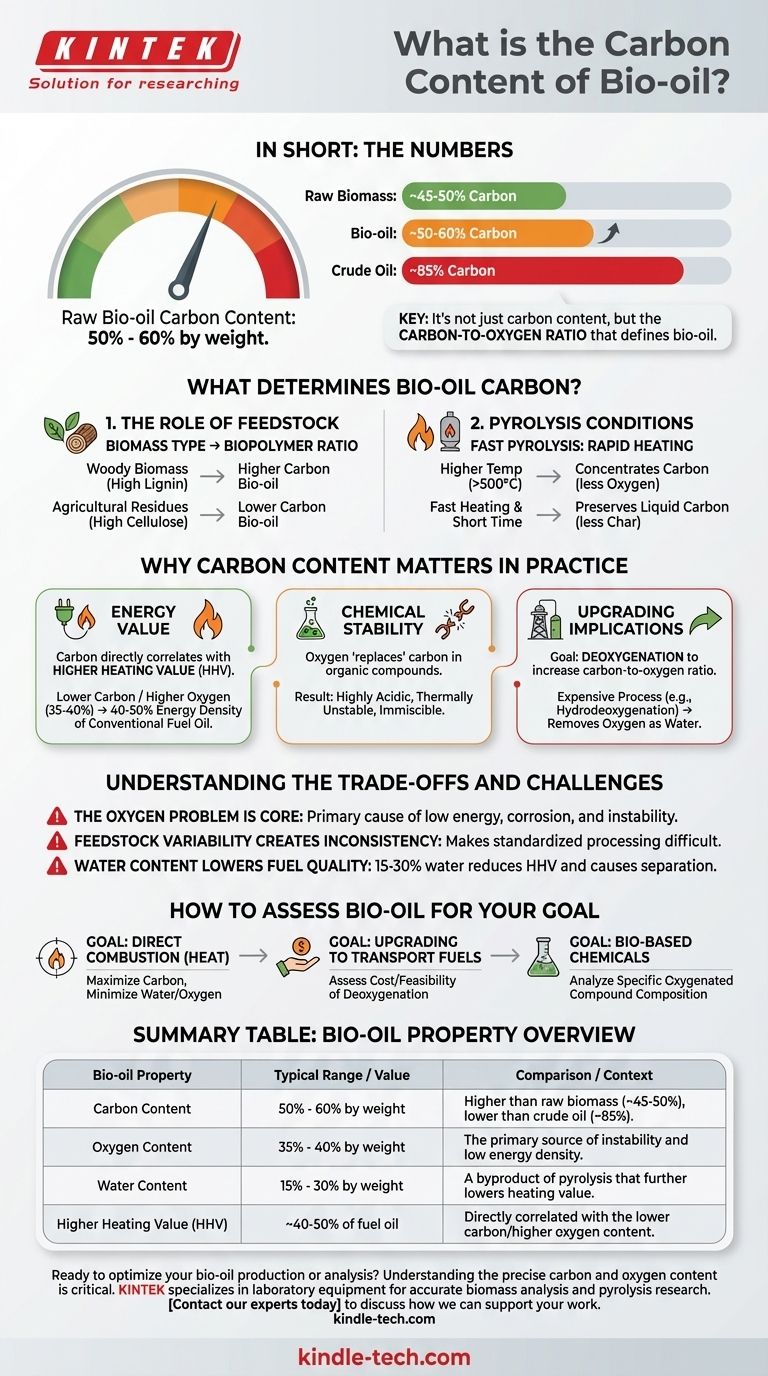
In short, the carbon content of raw bio-oil typically ranges from 50% to 60% by weight. This value is highly variable and represents a significant increase in carbon density compared to the original biomass (around 45-50%), but it remains substantially lower than that of conventional crude oil (around 85%).
The defining characteristic of bio-oil isn't just its carbon content, but its relationship to a very high oxygen content. Understanding this carbon-to-oxygen ratio is the key to evaluating its potential and its challenges as a renewable fuel or chemical feedstock.

What Determines the Carbon Content of Bio-oil?
The final carbon percentage in bio-oil is not a fixed number. It is the result of a complex interplay between the raw material you start with and the precise method used to convert it.
The Role of Feedstock
The chemical makeup of the starting biomass sets the initial baseline. Different plant materials have different ratios of key biopolymers.
For example, woody biomass is rich in lignin, a complex polymer with a higher carbon-to-oxygen ratio. Bio-oil produced from high-lignin feedstocks, like hardwoods or forestry residues, will generally have a higher carbon content.
Conversely, agricultural residues like grasses or straws are higher in cellulose and hemicellulose. These have more oxygen in their chemical structure, resulting in a bio-oil with a lower relative carbon content.
The Impact of Pyrolysis Conditions
Fast pyrolysis is the thermochemical process used to create bio-oil. It involves rapidly heating biomass in the absence of oxygen. The specific conditions of this process critically influence the final product.
- Temperature: Higher pyrolysis temperatures (e.g., >500°C) can promote secondary cracking reactions. This can break down larger molecules, potentially driving more oxygen out as water (H₂O) and carbon oxides (CO, CO₂), thereby concentrating the carbon in the remaining liquid oil.
- Heating Rate & Residence Time: Fast heating rates and short vapor residence times are the hallmarks of fast pyrolysis. This is crucial for maximizing liquid yield and preventing the bio-oil vapors from degrading into non-condensable gases and excess char, which preserves the carbon in the desired liquid product.
Why Carbon Content Matters in Practice
The percentage of carbon is a proxy for several of bio-oil's most important properties, dictating how it can be used and the challenges that must be overcome.
Impact on Energy Value
The most critical role of carbon is its direct correlation with the Higher Heating Value (HHV) of the fuel. Carbon and hydrogen are the primary elements that release energy during combustion.
Because bio-oil has a lower carbon content and a much higher oxygen content (35-40%) than fossil fuels, its energy density is significantly lower—roughly 40-50% that of conventional fuel oil.
Influence on Chemical Stability
The element that "replaces" carbon in bio-oil's makeup is oxygen. This high oxygen content is distributed across hundreds of different organic compounds, including acids, aldehydes, and ketones.
This makes raw bio-oil highly acidic (corrosive), thermally unstable (it can polymerize and thicken over time), and immiscible with hydrocarbon fuels.
Implications for Upgrading
To be used as a "drop-in" fuel for existing engines or refineries, bio-oil must be upgraded. The primary goal of upgrading is deoxygenation—removing oxygen atoms to increase the relative percentage of carbon and hydrogen.
Processes like hydrodeoxygenation add hydrogen under pressure to react with the oxygen, removing it as water. This is an expensive, energy-intensive process, but it is essential for producing a stable, high-energy-density hydrocarbon fuel from the initial bio-oil.
Understanding the Trade-offs and Challenges
While converting biomass to a carbon-dense liquid is a major step, the resulting bio-oil comes with inherent complications that must be addressed for practical application.
The Oxygen Problem is the Core Problem
The high oxygen content is the single greatest technical barrier to the widespread use of bio-oil. It is directly responsible for the fuel's low energy value, corrosivity, and instability. Every downstream challenge is, in some way, linked back to the presence of too much oxygen.
Feedstock Variability Creates Inconsistency
The dependency on feedstock type and process conditions means that bio-oil is not a standardized commodity like crude oil. This inconsistency makes it difficult to design and operate conversion and upgrading facilities that can handle a variable input while producing a consistent output.
Water Content Lowers Fuel Quality
Beyond the elemental composition, bio-oil also contains a significant amount of water (15-30%), which is a byproduct of the pyrolysis reactions. This water further reduces the heating value per unit of mass and can cause phase separation issues during storage.
How to Assess Bio-oil for Your Goal
Your evaluation of bio-oil's carbon content depends entirely on your intended application.
- If your primary focus is direct combustion for heat: Look for a bio-oil with the highest possible carbon content and lowest water and oxygen content to maximize its heating value (HHV).
- If your primary focus is upgrading to transportation fuels: The initial carbon content is less important than the feasibility and cost of deoxygenation to dramatically increase the carbon-to-oxygen ratio.
- If your primary focus is producing bio-based chemicals: The bulk carbon content is only a starting point; you must analyze the specific oxygenated chemical compounds that can be extracted as valuable platform chemicals.
Ultimately, understanding the factors that control bio-oil's carbon content is the first step toward engineering solutions that unlock its potential as a sustainable resource.
Summary Table:
| Bio-oil Property | Typical Range / Value | Comparison / Context |
|---|---|---|
| Carbon Content | 50% - 60% by weight | Higher than raw biomass (~45-50%), but lower than crude oil (~85%) |
| Oxygen Content | 35% - 40% by weight | The primary source of bio-oil's instability and low energy density |
| Water Content | 15% - 30% by weight | A byproduct of pyrolysis that further lowers heating value |
| Higher Heating Value (HHV) | ~40-50% of fuel oil | Directly correlated with the lower carbon/higher oxygen content |
Ready to optimize your bio-oil production or analysis?
Understanding the precise carbon and oxygen content of your bio-oil is critical for assessing its quality and potential applications. At KINTEK, we specialize in the laboratory equipment and consumables needed for accurate biomass analysis and pyrolysis research.
Whether you are developing new feedstocks, optimizing pyrolysis conditions, or analyzing the chemical composition of your bio-oil, our reliable tools can help you achieve consistent, high-quality results.
Contact our experts today to discuss how KINTEK can support your laboratory's work in renewable energy and sustainable materials.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Automatic Laboratory Heat Press Machine
- Three-dimensional electromagnetic sieving instrument
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
People Also Ask
- What role do laboratory autoclaves play in pectin extraction? Optimize Prebiotic Yield from Citrus and Apple Biomass
- What is the primary function of a laboratory autoclave in the pre-treatment of medical plastic waste for liquid fuel?
- What role does an autoclave play in the acid treatment for microalgae disruption? Unlock High-Yield Cell Pretreatment
- What are the advantages of using an autoclave equipped with a stirring device for molten salt testing? Dynamic Accuracy
- What is the necessity of using an autoclave for pre-treating culture media? Ensure Accurate Ag2O/TiO2 Testing








