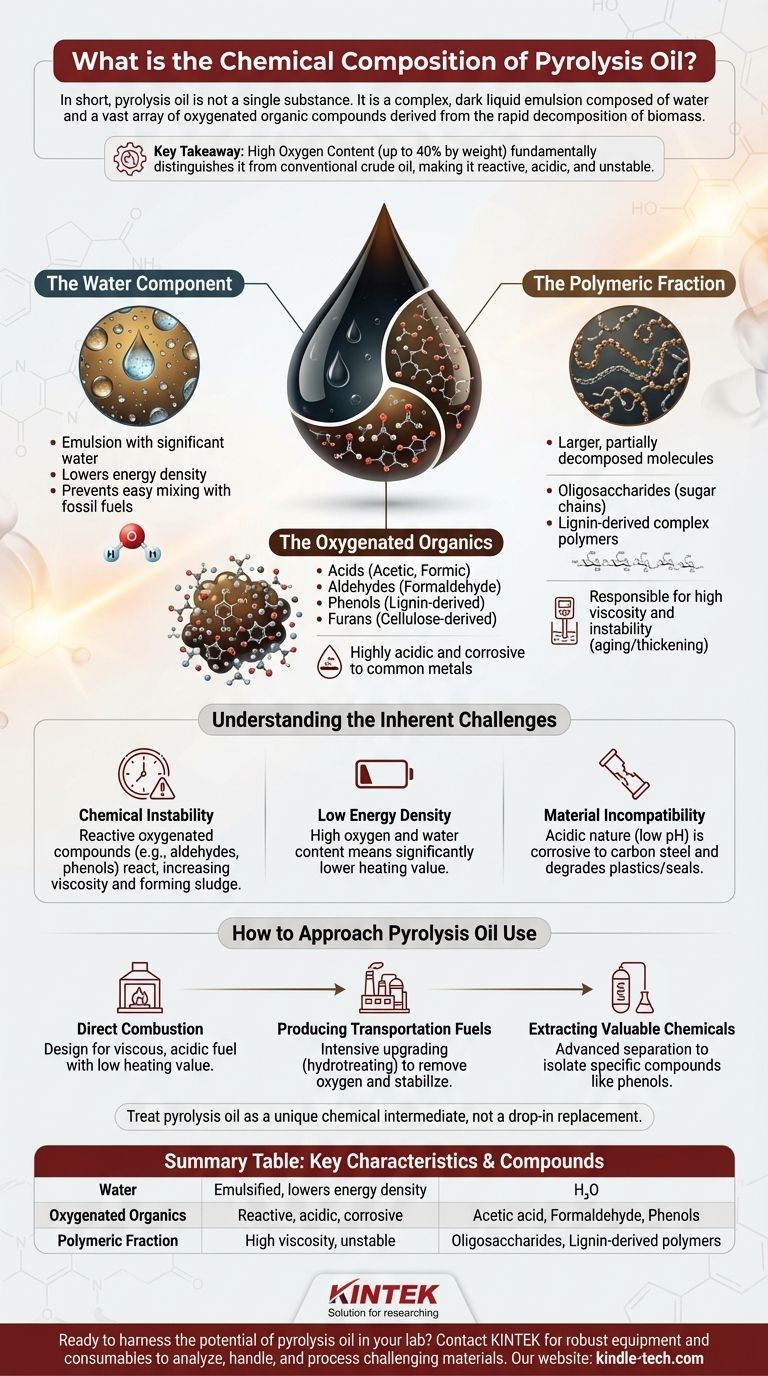
In short, pyrolysis oil is not a single substance. It is a complex, dark liquid emulsion composed of water and a vast array of oxygenated organic compounds derived from the rapid decomposition of biomass. This mixture contains everything from simple, low molecular weight chemicals like formaldehyde and acetic acid to large, high molecular weight polymers and oligosaccharides.
The key takeaway is that pyrolysis oil's high oxygen content, which can be up to 40% by weight, fundamentally distinguishes it from conventional crude oil. This oxygen is distributed across hundreds of different chemical compounds, making the oil reactive, acidic, and unstable.

Deconstructing the "Bio-Crude" Mixture
To understand pyrolysis oil, you must break it down into its primary chemical families. Its properties are a direct result of the interaction between these distinct components.
The Water Component
Pyrolysis oil is not pure oil; it is an emulsion that can contain a significant amount of water. This water is a byproduct of the pyrolysis reactions and is finely dispersed within the organic phase.
This water content lowers the overall energy density of the fuel and prevents it from easily mixing with conventional hydrocarbon fuels like diesel or gasoline.
The Oxygenated Organics
This is the largest and most complex fraction of pyrolysis oil. Unlike fossil fuels, which are almost entirely hydrocarbons, nearly every organic molecule in pyrolysis oil contains oxygen.
Key chemical groups include:
- Acids (like acetic and formic acid)
- Aldehydes (like formaldehyde)
- Phenols (derived from the breakdown of lignin)
- Furans (derived from the breakdown of cellulose)
The presence of these compounds, particularly the organic acids, makes the oil highly acidic and corrosive to common metals.
The Polymeric Fraction
Not all of the original biomass is broken down into simple molecules. A significant portion exists as larger, partially decomposed molecules.
This fraction includes oligosaccharides (chains of sugar molecules) and complex polymers derived from lignin. These heavy molecules are responsible for the oil's high viscosity and its tendency to thicken or "age" over time through re-polymerization.
Understanding the Inherent Challenges
The unique chemical composition of pyrolysis oil directly leads to several practical challenges that must be addressed before it can be used effectively.
Chemical Instability
The wide variety of reactive oxygenated compounds, such as aldehydes and phenols, makes the oil inherently unstable. Over time, these molecules can react with each other, causing the oil to increase in viscosity and even form solid sludge.
Low Energy Density
The high concentration of both oxygen and water means that pyrolysis oil has a significantly lower heating value (energy content) than conventional fossil fuels. More of its mass is non-combustible.
Material Incompatibility
Due to its acidic nature (low pH) and complex composition, pyrolysis oil is corrosive to many standard materials used for fuel storage and transport, such as carbon steel. It also degrades certain types of plastics and seals.
How to Approach Pyrolysis Oil Use
Understanding the chemical makeup is critical for determining how to handle and upgrade this potential resource. Your strategy must align with the oil's inherent properties.
- If your primary focus is direct combustion for energy: You must design systems that can handle a viscous, acidic fuel with a low heating value and account for its water content.
- If your primary focus is producing transportation fuels: You must plan for an intensive upgrading process, such as hydrotreating, to remove the high oxygen content and stabilize the molecules.
- If your primary focus is extracting valuable chemicals: You must utilize advanced separation and refining techniques to isolate specific compounds like phenols from the incredibly complex mixture.
Ultimately, treating pyrolysis oil not as a drop-in replacement for crude oil, but as a unique chemical intermediate, is the key to unlocking its value.
Summary Table:
| Component | Key Characteristics | Key Compounds |
|---|---|---|
| Water | Emulsified, lowers energy density | H₂O |
| Oxygenated Organics | Reactive, acidic, corrosive | Acetic acid, Formaldehyde, Phenols |
| Polymeric Fraction | High viscosity, unstable | Oligosaccharides, Lignin-derived polymers |
Ready to harness the potential of pyrolysis oil in your lab?
Understanding this complex bio-crude is the first step. KINTEK specializes in providing the robust lab equipment and consumables needed to safely analyze, handle, and process challenging materials like pyrolysis oil. From corrosion-resistant reactors to advanced separation tools, we help you turn chemical complexity into valuable insights and products.
Contact our experts today to discuss your specific laboratory needs and discover the right solutions for your research.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Heat Treat Sintering Brazing Furnace
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions


