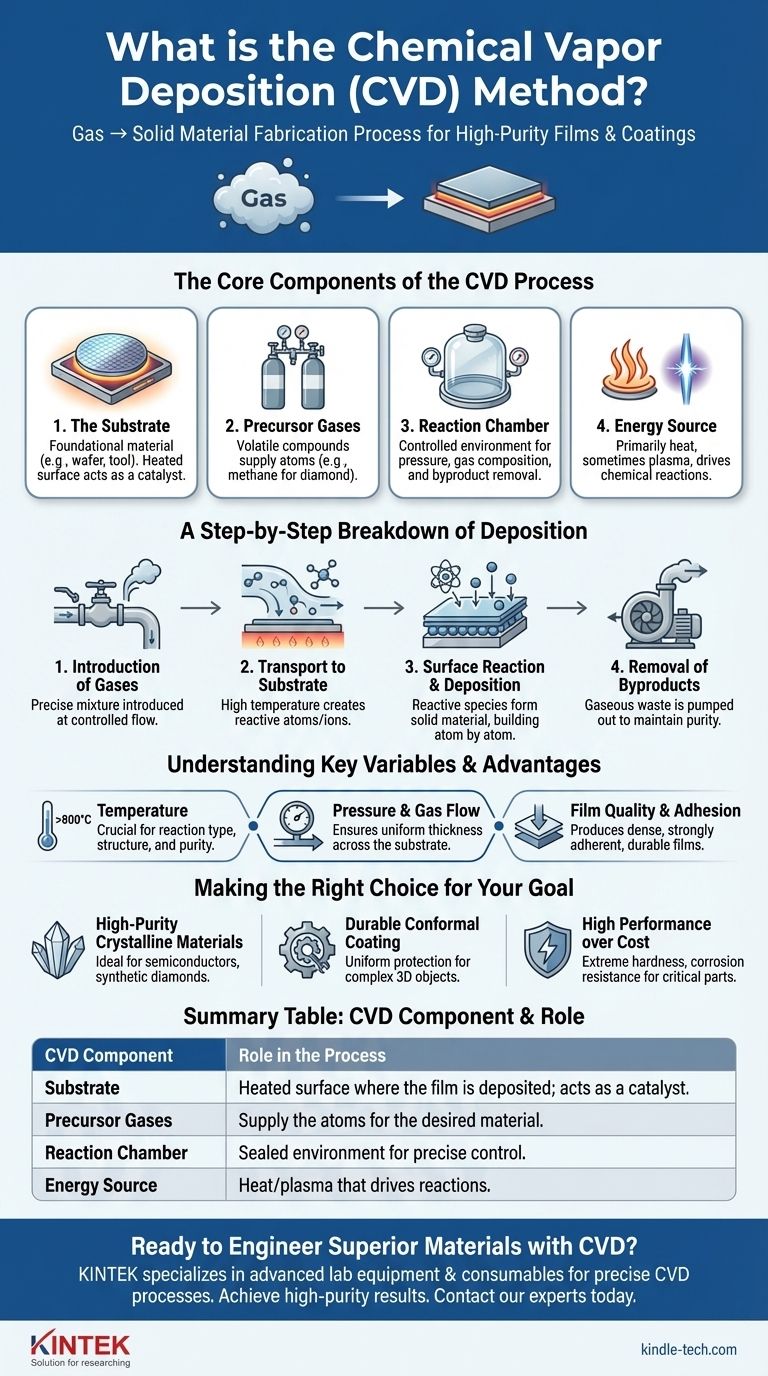
In essence, Chemical Vapor Deposition (CVD) is a material fabrication process used to create high-purity, high-performance solid films and coatings. It involves introducing precursor gases into a controlled chamber, where they undergo chemical reactions on a heated surface (known as a substrate) to deposit a thin, solid layer of the desired material.
The core principle of CVD is the transformation of a material from a gaseous state to a solid state through precisely controlled chemical reactions. The heated substrate is not just a surface for deposition; it is the catalyst and location for the chemical reaction that builds the film atom by atom.

The Core Components of the CVD Process
To understand how CVD works, it's essential to recognize its four main components, which work in concert to create the final product.
The Substrate
The substrate is the foundational material that will be coated. This can be anything from a silicon wafer for microelectronics to a cutting tool or even a tiny diamond seed crystal.
The substrate is heated to a specific, often very high temperature. This thermal energy is what drives the chemical reactions necessary for deposition. In many cases, the substrate surface itself acts as a catalyst for these reactions.
The Precursor Gases
These are volatile chemical compounds that contain the atoms of the material you want to deposit. For example, to create a diamond film, a carbon-rich gas like methane is used.
These precursors are often diluted with inert "carrier" gases that help transport them through the chamber at a controlled rate.
The Reaction Chamber
The entire process takes place within a sealed reaction chamber. This allows for precise control over the environment, including pressure, gas composition, and the removal of unwanted byproducts.
The Energy Source
While the heated substrate is the primary energy source, some CVD variations use additional energy to enhance the process.
This can include using microwaves or lasers to ionize the gases into a plasma, which breaks down the precursor molecules more efficiently and allows for deposition at lower temperatures.
A Step-by-Step Breakdown of Deposition
The CVD method follows a clear sequence of events to build a film on the substrate's surface.
1. Introduction of Gases
A precise mixture of precursor and carrier gases is introduced into the reaction chamber at a specified flow rate.
2. Transport to the Substrate
These gases flow over the heated substrate. The high temperature causes the gas molecules to decompose, creating highly reactive atoms, molecules, or ions.
3. Surface Reaction and Deposition
When these reactive species come into contact with the hot substrate, they undergo chemical reactions. The product of this reaction is a solid material that adheres to the surface.
This process builds up layer by layer, forming a solid film that is chemically bonded to the substrate.
4. Removal of Byproducts
The chemical reactions also create gaseous byproducts, which are effectively waste. These are continuously pumped out of the chamber to maintain a pure environment and drive the reaction forward.
Understanding the Trade-offs and Key Variables
CVD is a powerful but demanding technique. Its success depends entirely on meticulous process control.
The Critical Role of Temperature
The substrate's temperature is arguably the most crucial variable. It directly defines the type of chemical reactions that occur, which in turn determines the final film's structure, purity, and physical properties. Temperatures can often reach 800°C or higher.
Pressure and Gas Flow
The pressure inside the chamber and the flow rate of the gases must be managed precisely. These factors influence the uniformity of the coating, ensuring that the deposited film has a consistent thickness across the entire substrate.
Process Duration and Maintenance
CVD can be a very slow process. Creating a lab-grown diamond, for example, can take days or even weeks. During long runs, the process may need to be stopped periodically for maintenance, such as removing unwanted material deposits from chamber walls.
Film Quality and Adhesion
A primary advantage of CVD is its ability to produce dense, adherent films. Because the coating is grown through a chemical reaction on the surface, it forms a strong bond with the substrate, resulting in a highly durable layer.
Making the Right Choice for Your Goal
CVD is not a one-size-fits-all solution. Its application is best suited for specific manufacturing and engineering objectives where material quality is paramount.
- If your primary focus is creating high-purity, crystalline materials: CVD is ideal for applications like semiconductor manufacturing or growing synthetic diamonds, as the slow, controlled deposition allows atoms to arrange into a stable crystal lattice.
- If your primary focus is applying a durable, conformal coating: CVD excels at coating complex 3D objects, as the gaseous precursors can access and react on all exposed surfaces to form a uniform, protective layer.
- If your primary focus is on performance over cost for a critical component: CVD is the method of choice for creating coatings that provide extreme hardness, corrosion resistance, or specific electronic properties, even though the equipment and process can be complex.
By mastering the interplay of gas, heat, and chemistry, the CVD method provides a powerful tool for engineering materials from the atom up.
Summary Table:
| CVD Component | Role in the Process |
|---|---|
| Substrate | Heated surface where the film is deposited; acts as a catalyst. |
| Precursor Gases | Supply the atoms for the desired material (e.g., methane for diamond). |
| Reaction Chamber | Sealed environment for precise control of pressure and gas composition. |
| Energy Source | Heat (and sometimes plasma) that drives the chemical reactions. |
| Key Advantages | High Purity, Conformal Coating, Strong Adhesion, Dense Films |
Ready to Engineer Superior Materials with CVD?
KINTEK specializes in the advanced lab equipment and consumables required for precise Chemical Vapor Deposition processes. Whether you are developing semiconductors, creating durable protective coatings, or growing synthetic diamonds, our solutions help you achieve the high-purity, high-performance results your research demands.
Contact our experts today via our form to discuss how we can support your specific laboratory and manufacturing goals.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is CVD method for graphene? A Scalable Process for High-Quality, Large-Area Films
- What is the purpose of creating thin films? Unlock New Surface Properties for Your Materials
- What gases are typically used in the High-Density Plasma CVD (HDP-CVD) process? Optimize Your Film Deposition
- What is the deposition rate of sputtering? A Guide to Controlling Your Thin Film Process
- Why is CVD the most efficient method for preparing graphene? Unlock Scalable, High-Quality Material Production
- What is the process of chemical Vapour deposition CVD? A Step-by-Step Guide to High-Purity Film Growth
- What is deposition in nanotechnology? Build High-Performance Materials Atom by Atom
- What unique role does an i-CVD system play in 3D structure modification? Achieve Precision Super-Amphiphobicity



















