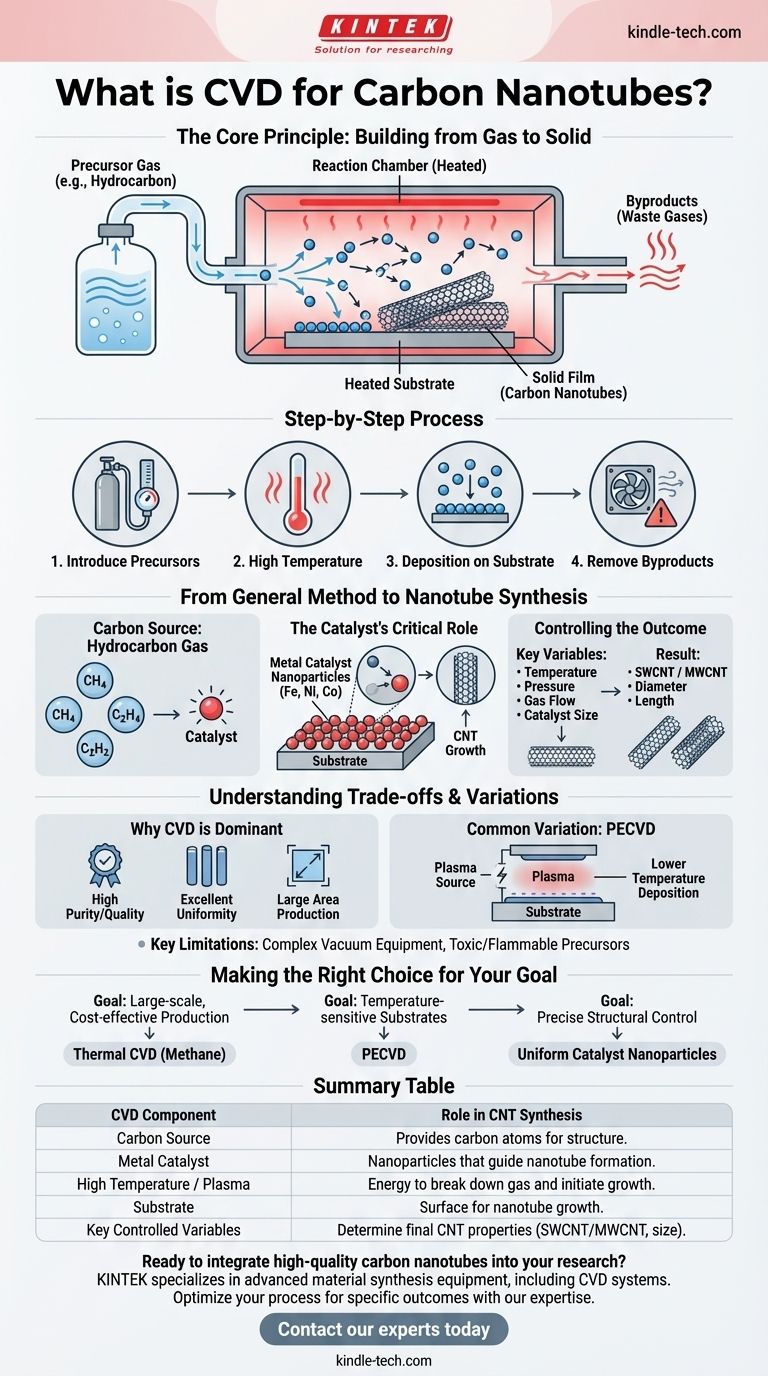
In essence, Chemical Vapor Deposition (CVD) is a method for building a solid material from a gas. It involves introducing one or more volatile precursor gases into a reaction chamber containing a heated substrate. The high temperature causes chemical reactions that break down the gases, depositing a thin film of solid material onto the substrate's surface, while any unwanted byproducts are exhausted.
The core principle of CVD is transforming gas-phase molecules into a high-performance solid film. For carbon nanotubes, this process is adapted by using a carbon-based gas and a metal catalyst to guide the assembly of carbon atoms into the unique nanotube structure.

The Core Principle: Building from Gas to Solid
Chemical Vapor Deposition is a "bottom-up" manufacturing technique, meaning it constructs materials atom by atom or molecule by molecule. The entire process hinges on a controlled chemical reaction in a specialized environment.
Step 1: Introducing the Precursors
The process begins by feeding precise amounts of precursor gases into a reaction chamber. These precursors are volatile compounds that contain the elements needed for the final solid material.
Step 2: The Role of High Temperature
Inside the chamber, a substrate (the surface where the film will grow) is heated to very high temperatures. This thermal energy is the catalyst that breaks apart the chemical bonds in the precursor gas molecules.
Step 3: Deposition on the Substrate
Once the precursor molecules decompose, the desired atoms or molecules settle onto the hot substrate. There, they react and bond with each other, gradually forming a stable and uniform solid film.
Step 4: Removing the Byproducts
The chemical reactions also create gaseous byproducts that are not part of the final film. These waste gases are safely vented out of the reaction chamber, leaving behind only the pure, solid material.
From General Method to Nanotube Synthesis
While the general principles of CVD apply, creating carbon nanotubes (CNTs) requires specific ingredients and conditions. The process is carefully tuned to encourage carbon atoms to assemble in a cylindrical, graphitic structure.
The Carbon Source
Instead of a generic precursor, a hydrocarbon gas is used as the carbon source. Common examples include methane (CH₄), ethylene (C₂H₄), or acetylene (C₂H₂). When heated, these gases release carbon atoms.
The Catalyst's Critical Role
This is the most crucial adaptation for CNT growth. The substrate is coated with a thin layer of nanoparticle metal catalysts, typically iron (Fe), nickel (Ni), or cobalt (Co). The carbon atoms from the precursor gas dissolve into these heated metal particles and then precipitate out to form the cylindrical walls of the nanotube.
Controlling the Outcome
The final structure of the nanotubes—whether they are single-walled (SWCNT) or multi-walled (MWCNT), their diameter, and their length—is determined by precisely controlling the experimental conditions. Key variables include temperature, pressure, gas flow rates, and the size of the catalyst particles.
Understanding the Trade-offs and Variations
CVD is a powerful and widely used technique, but it is essential to understand its context, including its advantages and common adaptations.
Why CVD is a Dominant Method
CVD is favored for its ability to produce high-quality, high-purity films with excellent uniformity over large areas. This makes it an industrially significant process for everything from microelectronics to advanced materials like CNTs.
Common Variation: Plasma-Enhanced CVD (PECVD)
A key limitation of traditional thermal CVD is the requirement for very high temperatures, which can damage sensitive substrates. Plasma-Enhanced CVD (PECVD) uses an electric field to generate a plasma, which provides the energy to break down the precursor gases. This allows deposition to occur at much lower temperatures.
Key Limitations to Consider
The primary drawbacks of CVD methods can include the complexity and cost of the required vacuum equipment. Furthermore, many precursor gases are toxic, flammable, or corrosive, necessitating stringent safety protocols.
Making the Right Choice for Your Goal
The versatility of CVD allows it to be adapted for different objectives. Your specific goal will determine which process parameters are most critical.
- If your primary focus is large-scale, cost-effective production: Thermal CVD with a common hydrocarbon like methane is a robust and well-understood starting point.
- If your primary focus is growing nanotubes on a temperature-sensitive polymer substrate: PECVD is the necessary choice to avoid damaging the underlying material.
- If your primary focus is precise structural control (e.g., specific diameters): Your efforts should concentrate on fabricating catalyst nanoparticles of a highly uniform and specific size.
Ultimately, mastering CVD for carbon nanotube synthesis is about the precise control of chemistry and energy to build a remarkable material from the ground up.
Summary Table:
| CVD Component | Role in CNT Synthesis |
|---|---|
| Carbon Source (e.g., Methane) | Provides the carbon atoms that form the nanotube structure. |
| Metal Catalyst (e.g., Iron, Nickel) | Nanoparticles that dissolve carbon and guide the formation of cylindrical nanotubes. |
| High Temperature / Plasma | Provides energy to break down the gas molecules and initiate growth. |
| Substrate | The surface on which the carbon nanotubes grow. |
| Key Controlled Variables | Temperature, pressure, gas flow rates, and catalyst size determine the final CNT properties (SWCNT/MWCNT, diameter, length). |
Ready to integrate high-quality carbon nanotubes into your research or product development? The CVD process requires precise control and reliable equipment to achieve consistent results. KINTEK specializes in lab equipment and consumables for advanced material synthesis, including CVD systems. Our expertise can help you optimize your process for specific outcomes, whether you need large-scale production or growth on sensitive substrates. Contact our experts today to discuss how we can support your laboratory's innovation in nanotechnology.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What equipment is used to grow lab diamonds? HPHT & CVD Diamond Growth Systems Explained
- What are the primary disadvantages of Chemical Vapor Deposition (CVD)? Navigate Challenges in Thin Film Manufacturing
- What is MOCVD equipment? The Key to Growing High-Performance Semiconductor Crystals
- What is deposition in the semiconductor industry? The Foundational Process for Building Microchips
- What is the process gas for sputtering? Optimize Your Thin Film Deposition with the Right Gas
- What is the sputtering cathode method? A Guide to Thin Film Deposition Technology
- What is the difference between CVD and MOCVD? Precision vs. Versatility in Thin-Film Deposition
- What is sublimation and deposition in chemistry? Mastering Solid-Gas Phase Transitions



















