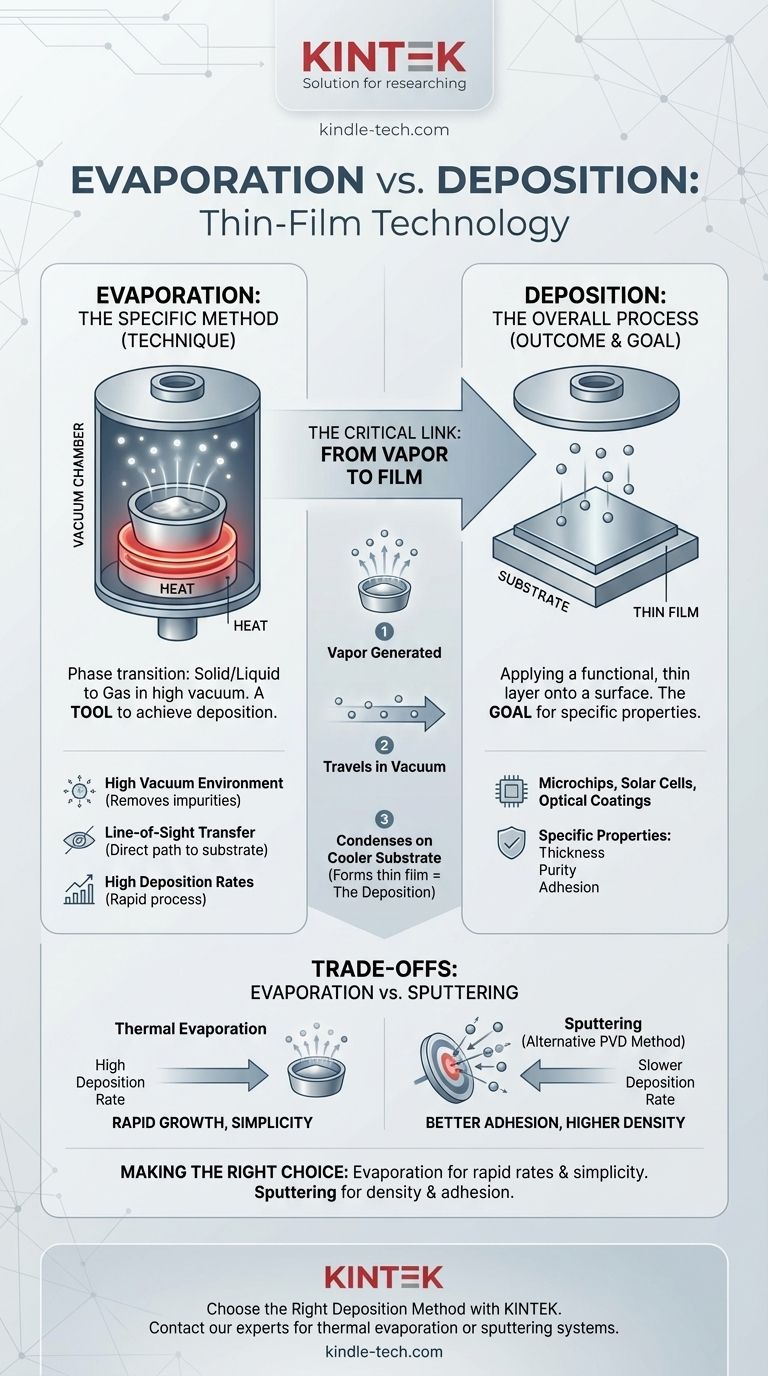
In the context of material science and manufacturing, evaporation is a specific method used to achieve the broader process of deposition. Deposition is the general term for applying a thin film of material onto a surface (a substrate), while thermal evaporation is one of the primary techniques used to create the material vapor that forms that film.
The core distinction is one of process versus outcome. Deposition is the outcome of adding a thin layer to a surface, whereas evaporation is a specific physical mechanism—heating a material in a vacuum until it becomes a gas—used to enable that deposition.
Defining the Core Concepts
To understand the relationship between these terms, it's essential to define each one clearly within the context of thin-film technology.
What is Deposition?
Deposition is the foundational process of adding a functional, thin layer of material onto a substrate. This is a fundamental step in manufacturing high-tech components like microchips, solar cells, and optical coatings. The goal of deposition is to create a film with specific properties, such as thickness, purity, and adhesion.
What is Evaporation?
Evaporation is the phase transition of a substance from a solid or liquid state into a gaseous state. In the manufacturing process known as thermal evaporation, a source material (like aluminum or gold) is heated in a high-vacuum chamber until its atoms vaporize.
The Critical Link: From Vapor to Film
The connection between the two terms is direct and sequential. The vapor generated by the evaporation process travels through the vacuum chamber. When these gaseous atoms or molecules strike the cooler surface of the substrate, they condense back into a solid state, forming a thin, uniform film. This act of condensing and building the film is the deposition.
The Mechanics of Thermal Evaporation Deposition
The process of using evaporation for deposition is conceptually straightforward, relying on a few key principles to ensure a high-quality result.
The Role of the Vacuum
A high-vacuum environment is critical. It removes air and other unwanted gas molecules that could otherwise react with the hot vapor or interfere with its path to the substrate. This ensures the purity of the deposited film.
Line-of-Sight Transfer
Inside the vacuum, the evaporated particles travel in a straight line from the source to the substrate. This is often referred to as a "line-of-sight" process, as nothing obstructs the path of the vapor.
High Deposition Rates
As the source material is heated, it can produce a very dense vapor stream. This allows for a high volume of material to be transferred to the substrate quickly, resulting in high deposition rates and relatively short processing times.
Understanding the Trade-offs: Evaporation vs. Other Methods
Evaporation is a powerful technique, but it is just one of several methods used for physical vapor deposition (PVD). Understanding its place among alternatives clarifies its advantages and limitations.
The Alternative: Sputtering
Another common PVD method is sputtering. Instead of heating a material, sputtering uses high-energy ions to bombard a source target, physically knocking off atoms that then deposit onto the substrate.
Speed vs. Control
Compared to thermal evaporation, sputtering generally has lower deposition rates. However, the sputtering process can sometimes offer better film adhesion and density because the ejected atoms have higher kinetic energy when they strike the substrate. The choice between them depends entirely on the requirements of the final product.
Making the Right Choice for Your Goal
Selecting the correct deposition method depends on the desired properties of the thin film and the efficiency requirements of the manufacturing process.
- If your primary focus is rapid film growth and simplicity: Thermal evaporation is often the ideal choice due to its characteristically high deposition rates.
- If your primary focus is film density, adhesion, or depositing complex alloys: A method like sputtering might be more suitable, despite its typically slower process time.
Ultimately, recognizing that evaporation is a tool to achieve the goal of deposition is the key to selecting the right technique for your application.
Summary Table:
| Aspect | Deposition | Evaporation (Thermal) |
|---|---|---|
| Definition | The overall process of applying a thin film onto a substrate. | A specific method of vaporizing a source material to enable deposition. |
| Role | The desired outcome or goal. | A technique used to achieve the outcome. |
| Key Characteristic | Creates a functional layer with specific properties. | Uses heat in a vacuum to create a vapor. |
| Process Speed | Varies by method. | Typically high deposition rates. |
| Film Quality | Depends on the method used (e.g., adhesion, density). | Good for simplicity and speed; sputtering may offer better adhesion. |
Ready to Choose the Right Deposition Method for Your Lab?
Understanding the nuances between evaporation and other deposition techniques is critical for achieving the perfect thin film for your application—whether it's for microchips, solar cells, or optical coatings.
KINTEK specializes in lab equipment and consumables, serving all your laboratory needs. Our experts can help you select the ideal thermal evaporation or sputtering system to ensure high purity, excellent adhesion, and maximum efficiency for your research and production goals.
Contact us today via our [#ContactForm] to discuss your specific requirements and discover how KINTEK's solutions can enhance your thin-film manufacturing process.
Visual Guide
Related Products
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Vacuum Cold Trap Direct Cold Trap Chiller
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
People Also Ask
- What is thermal evaporation of thin film? A Guide to High-Purity PVD Coating
- What type of deposition is resulted at high vacuum? Achieve Pure, High-Performance Thin Films with PVD
- What is the electron beam physical vapor deposition process? A Guide to High-Purity Thin Films
- What are thermal evaporation sources? Key Types and How to Choose the Right One
- Which parameter effect on thin film formation in thermal evaporation? Master the Key Variables for Superior Films
- How thick is the film in e-beam evaporation? Achieve Precise Control from Nanometers to Micrometers
- What are the advantages of thermal evaporation? Fast, Low-Cost Thin Film Deposition
- What is the electron beam method? A Guide to Precision Coating, Cutting & Sterilization



















