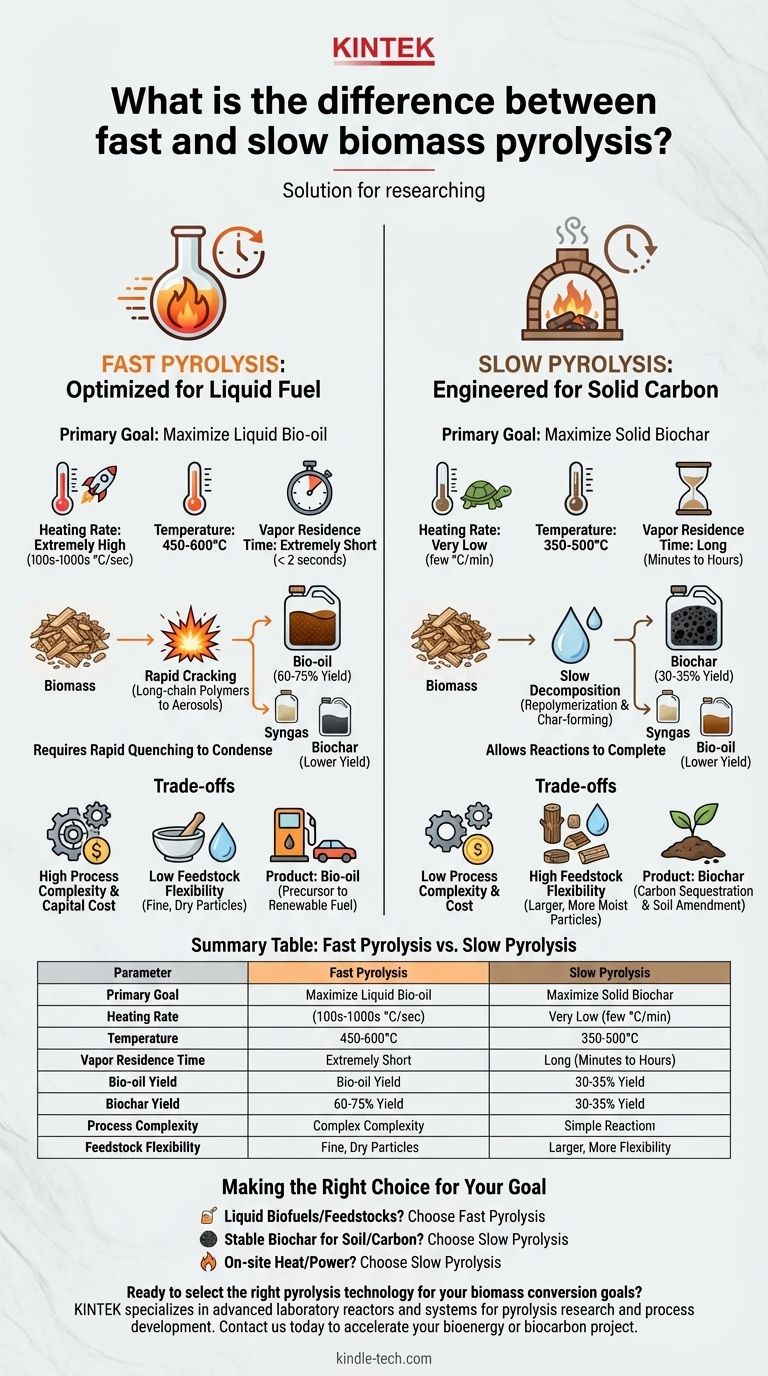
The primary difference between fast and slow biomass pyrolysis lies in the process conditions—specifically the heating rate, temperature, and vapor residence time. These parameters are deliberately controlled to fundamentally alter the chemical reactions and, as a result, determine the primary output. Fast pyrolysis is engineered to maximize liquid bio-oil production, while slow pyrolysis is optimized to yield solid biochar.
The choice between fast and slow pyrolysis is not a matter of speed, but a strategic decision based on your desired end product. Fast pyrolysis targets liquid fuel; slow pyrolysis targets solid carbon.

Deconstructing Pyrolysis
What is Pyrolysis?
Pyrolysis is the thermal decomposition of organic material, such as biomass, at elevated temperatures in the near-complete absence of oxygen.
Instead of burning the material, this process breaks down complex hydrocarbon molecules into three distinct products: a liquid (bio-oil), a solid (biochar), and a non-condensable gas (syngas).
The Three Key Control Parameters
The final yields of these three products are directly controlled by three main process variables.
- Heating Rate: How quickly the biomass temperature is raised.
- Temperature: The final temperature the biomass reaches inside the reactor.
- Residence Time: How long the biomass solids and vapor products remain at the reaction temperature.
By manipulating these "knobs," we can favor the formation of one product over the others.
Fast Pyrolysis: Optimized for Liquid Fuel
The Process Conditions
Fast pyrolysis uses extremely high heating rates (hundreds or thousands of degrees Celsius per second) and moderate temperatures, typically between 450-600°C.
Critically, the vapor residence time is kept extremely short, usually less than two seconds. This requires rapidly quenching or cooling the hot vapors to condense them into a liquid.
The Chemical Mechanism
The rapid heating cracks the long-chain polymers in biomass (like cellulose and lignin) into smaller, aerosol-sized molecules.
The very short vapor residence time is essential. It removes these valuable vapor molecules from the hot reaction zone before they can undergo secondary reactions, which would otherwise convert them into more stable char or lighter gases.
Primary Product: Bio-oil
This process maximizes the yield of liquid bio-oil, often achieving 60-75% of the product mass.
Bio-oil is a dense, dark-brown liquid that can be viewed as a precursor to renewable gasoline or diesel. However, it is acidic, unstable, and has a high oxygen content, requiring significant and costly upgrading before it can be used as a drop-in fuel.
Slow Pyrolysis: Engineered for Solid Carbon
The Process Conditions
Slow pyrolysis, historically known as charcoal making, uses very low heating rates (a few degrees Celsius per minute) and lower-to-moderate temperatures, typically 350-500°C.
The process is defined by a very long residence time, lasting from many minutes to several hours.
The Chemical Mechanism
The slow heating and long residence time allow the decomposition reactions to proceed to completion. This environment promotes secondary char-forming reactions and repolymerization, where volatile compounds convert into more stable, aromatic carbon structures.
This process systematically drives off volatile components, leaving behind a fixed carbon skeleton.
Primary Product: Biochar
This process is designed to maximize the yield of solid biochar, typically achieving around 30-35% of the product mass.
Biochar is a stable, highly porous, and carbon-rich material. It is primarily used for carbon sequestration, as a soil amendment to improve fertility and water retention, or in filtration applications.
Understanding the Trade-offs
Product Yield vs. Process Complexity
Fast pyrolysis offers a high yield of a high-value liquid product, but it demands sophisticated engineering to manage the rapid heat transfer and short residence times.
Slow pyrolysis is a much simpler, more robust, and lower-tech process, but its primary product, biochar, generally has a lower market value than liquid fuels.
Feedstock Requirements
Fast pyrolysis is highly sensitive to feedstock preparation. It requires finely ground particles (typically <2 mm) and very low moisture content (<10%) to ensure rapid heat transfer.
Slow pyrolysis is far more forgiving. It can handle larger, non-uniform particles and higher moisture content, reducing the cost and energy spent on pre-processing the biomass.
Capital and Operating Costs
The precise engineering required for fast pyrolysis reactors generally results in higher capital investment and operating costs.
Slow pyrolysis systems can be built and operated much more simply and cheaply, making them more accessible for smaller-scale or decentralized applications.
Making the Right Choice for Your Goal
The decision to use fast or slow pyrolysis is a direct function of your strategic objective.
- If your primary focus is producing liquid biofuels or renewable chemical feedstocks: Fast pyrolysis is the only viable pathway to maximize bio-oil yield.
- If your primary focus is creating stable biochar for carbon sequestration or soil improvement: Slow pyrolysis is the superior and more efficient method.
- If your primary focus is generating on-site heat and power from waste biomass with simpler technology: Slow pyrolysis provides a stable solid fuel (biochar) and a combustible syngas that can be used directly.
Ultimately, understanding these core differences allows you to align the process technology directly with your desired end product.
Summary Table:
| Parameter | Fast Pyrolysis | Slow Pyrolysis |
|---|---|---|
| Primary Goal | Maximize Liquid Bio-oil | Maximize Solid Biochar |
| Heating Rate | Very High (100s-1000s °C/sec) | Very Low (few °C/min) |
| Temperature | 450-600°C | 350-500°C |
| Vapor Residence Time | Very Short (< 2 seconds) | Long (minutes to hours) |
| Typical Bio-oil Yield | 60-75% | Lower |
| Typical Biochar Yield | Lower | 30-35% |
| Process Complexity | High (requires rapid quenching) | Low (simpler technology) |
| Feedstock Flexibility | Low (requires fine, dry particles) | High (forgiving of moisture/particle size) |
Ready to select the right pyrolysis technology for your biomass conversion goals?
Whether your project targets high-yield bio-oil for renewable fuels or stable biochar for carbon sequestration and soil enhancement, having the right equipment is critical. KINTEK specializes in advanced laboratory reactors and systems for pyrolysis research and process development.
We provide the precise, reliable equipment you need to test and optimize your specific biomass feedstocks and process parameters.
Contact us today using the form below to discuss how our solutions can accelerate your bioenergy or biocarbon project. #ContactForm
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Graphite Vacuum Continuous Graphitization Furnace
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
People Also Ask
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
















