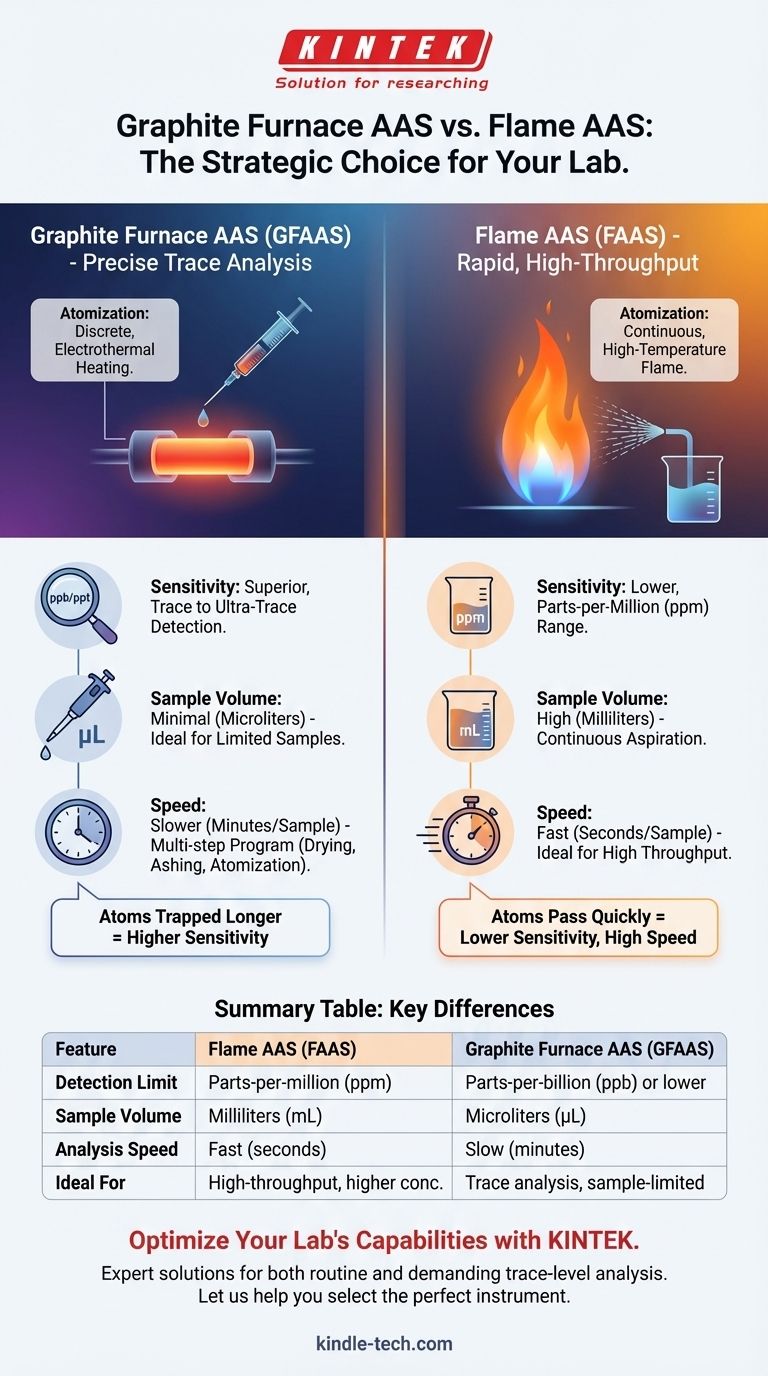
The fundamental difference between Graphite Furnace AAS (GFAAS) and Flame AAS (FAAS) is the method used to convert the sample into free, ground-state atoms for analysis. FAAS uses a high-temperature flame to continuously atomize a liquid sample, while GFAAS uses an electrically heated graphite tube to discretely atomize a very small, specific volume of the sample. This core difference in atomization dictates their respective performance, sensitivity, and ideal applications.
The choice between FAAS and GFAAS is a strategic decision driven by your analytical needs. FAAS offers speed and simplicity for higher concentrations (ppm range), while GFAAS provides superior sensitivity (ppb range) for trace analysis and works with minimal sample volumes.

The Fundamental Difference: The Atomization Process
Atomic Absorption Spectroscopy (AAS) works by measuring the light absorbed by free atoms. To do this, the element of interest within a sample must first be liberated from its chemical bonds and converted into an atomic vapor, a process called atomization.
Flame AAS (FAAS): Continuous Atomization in a Flame
In FAAS, the liquid sample is continuously drawn up (aspirated) and sprayed as a fine mist into a long, narrow flame.
The high temperature of the flame (typically 2000-3000°C) serves to rapidly desolvate, vaporize, and atomize the elements. The light beam from the source lamp passes through this flame, and the instrument measures the absorption by the atoms as they briefly pass through the light path.
Graphite Furnace AAS (GFAAS): Discrete Atomization in a Tube
In GFAAS, a very small, discrete volume of sample (typically 5-50 microliters) is precisely injected into a small graphite tube.
This tube is then heated electrothermally in a programmed sequence of steps:
- Drying: Low temperature to gently evaporate the solvent.
- Ashing (Pyrolysis): Medium-high temperature to burn off organic matrix components.
- Atomization: A very rapid temperature ramp to >2000°C to vaporize and atomize the analyte.
The atoms are momentarily trapped inside the confined space of the tube, significantly increasing the time they spend in the instrument's light path.
Key Performance Differences Explained
The difference in atomization directly leads to critical differences in analytical performance.
Sensitivity and Detection Limits
GFAAS is vastly more sensitive than FAAS. It can achieve detection limits 100 to 1,000 times lower, often reaching parts-per-billion (ppb) or even parts-per-trillion (ppt) levels.
This is because the entire injected sample is atomized, and the atoms are concentrated in a small volume for a longer duration. In FAAS, most of the sample goes to waste, and atoms pass through the flame very quickly.
Sample Volume
GFAAS is the ideal technique for sample-limited analysis. It requires only microliters (µL) of sample per analysis.
FAAS, by contrast, is a sample-intensive technique. It requires continuous aspiration, consuming several milliliters (mL) of sample to get a stable reading.
Speed and Throughput
FAAS is significantly faster than GFAAS. Once the instrument is calibrated, a single sample analysis can take as little as 10-15 seconds. This makes it ideal for laboratories with a high sample throughput.
A single GFAAS analysis takes several minutes due to the multi-step heating program. This low throughput makes it unsuitable for routine analysis of a large number of samples.
Understanding the Trade-offs
Choosing between these techniques involves balancing sensitivity against speed, cost, and complexity.
The Cost of Sensitivity (GFAAS)
While powerful, GFAAS is slower and more expensive. The graphite tubes are consumable parts with a finite lifetime (hundreds of firings) and must be replaced regularly, adding to operational costs. Method development can also be more complex, requiring careful optimization of the temperature program to manage matrix interferences.
The Simplicity of Speed (FAAS)
FAAS is robust, simple to operate, and has lower running costs. Its speed makes it highly efficient for analyzing many samples for elements present at the parts-per-million (ppm) level or higher. However, its lower sensitivity makes it completely ineffective for trace or ultra-trace analysis.
The Challenge of Interferences
Both techniques are subject to interferences. GFAAS can be more susceptible to background absorption from the sample matrix being vaporized in the furnace. Modern instruments use powerful background correction techniques (like Zeeman correction) to mitigate this. FAAS is less prone to background issues but can suffer from chemical interferences in the flame, which are managed with different strategies.
Choosing the Right Technique for Your Analysis
Your analytical goal is the only factor that matters when selecting a technique.
- If your primary focus is high throughput and percent-to-ppm level concentrations: Choose Flame AAS for its speed, simplicity, and lower operational cost.
- If your primary focus is trace or ultra-trace analysis (ppm-to-ppb levels): Choose Graphite Furnace AAS for its superior sensitivity and analytical power.
- If you are analyzing precious or volume-limited samples: GFAAS is the only viable option due to its requirement for only microliters of sample.
Understanding these core differences ensures you select not just a different instrument, but the correct analytical strategy for your specific goal.
Summary Table:
| Feature | Flame AAS (FAAS) | Graphite Furnace AAS (GFAAS) |
|---|---|---|
| Detection Limit | Parts-per-million (ppm) | Parts-per-billion (ppb) or lower |
| Sample Volume | Milliliters (mL) | Microliters (µL) |
| Analysis Speed | Fast (seconds per sample) | Slow (minutes per sample) |
| Ideal For | High-throughput, higher concentration analysis | Trace analysis, sample-limited applications |
Optimize your lab's analytical capabilities with the right AAS solution from KINTEK.
Whether your priority is high-throughput analysis of major elements or sensitive detection of trace metals, choosing the correct Atomic Absorption Spectroscopy technique is critical for accurate and efficient results. KINTEK specializes in providing high-quality lab equipment and consumables, including robust Flame AAS systems for routine analysis and sensitive Graphite Furnace AAS systems for demanding trace-level work.
Let our experts help you select the perfect instrument to meet your specific sensitivity, sample volume, and throughput requirements.
Contact KINTEK today to discuss your laboratory needs and enhance your analytical precision.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Is graphite good in high temperature? Unlocking Its Extreme Heat Potential
- What is the graphite furnace used for? Achieve Extreme Heat Up to 3000°C in a Controlled Environment
- What are the properties of graphite at high temperatures? Unlock Its Strength and Stability in Extreme Heat
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- What is the thermal limit of graphite? Unlock Extreme Heat Performance in Your Lab



















