While incredibly powerful, the primary disadvantage of a graphite furnace is the inherent reactivity and consumable nature of graphite itself. At high temperatures, graphite readily oxidizes in the presence of air and can introduce carbon into the process atmosphere, which can be a significant source of contamination for many materials. This necessitates a strictly controlled vacuum or inert gas environment to function.
The core trade-off of a graphite furnace is that its greatest strength—graphite's ability to heat rapidly and efficiently—is also the source of its main weaknesses: a limited lifespan and the potential for carbon contamination.

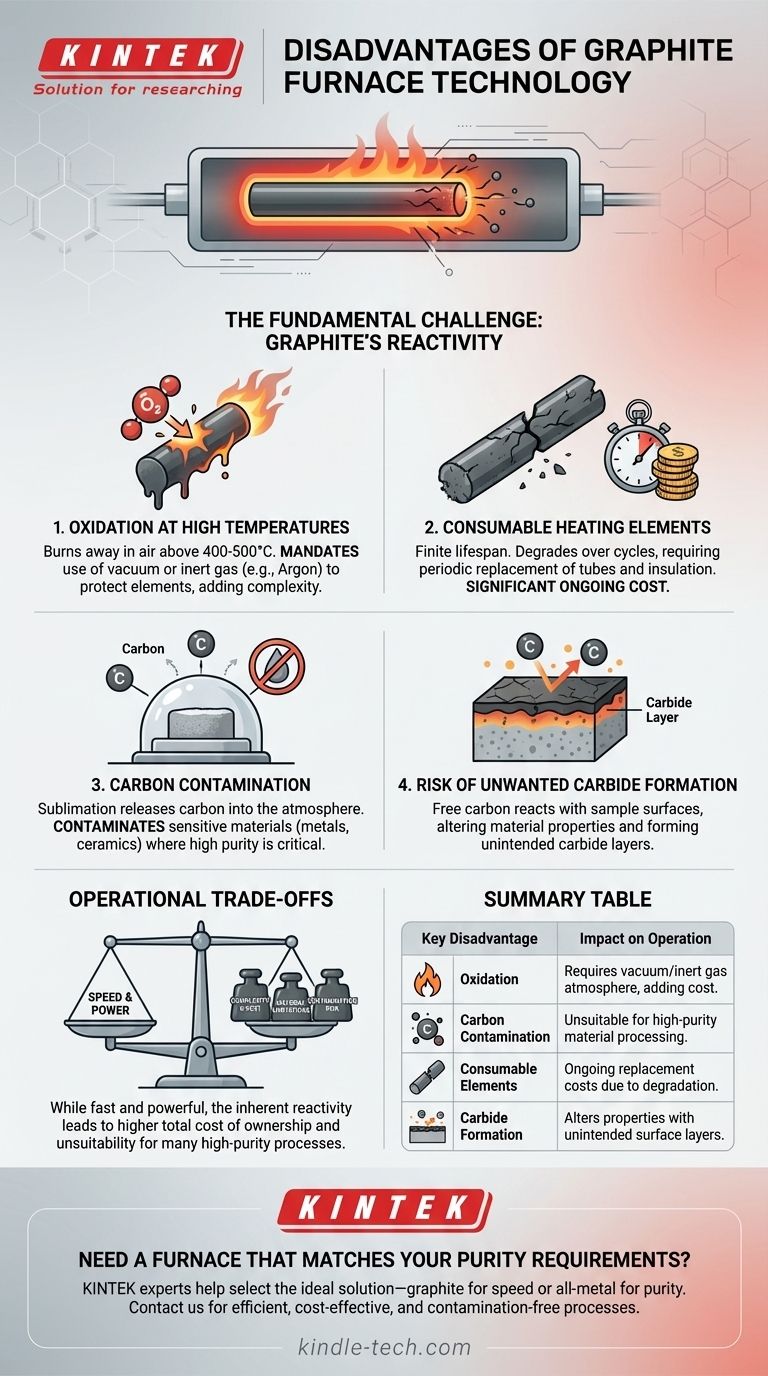
The Fundamental Challenge: Graphite's Reactivity
The defining limitation of a graphite furnace stems from the chemical properties of its core heating element. While the references highlight its excellent thermo-mechanical properties, these come with significant operational constraints.
Oxidation at High Temperatures
Graphite is a form of carbon. When heated to high temperatures (typically above 400-500°C) in the presence of oxygen, it will literally burn away.
This makes operating a graphite furnace in an air atmosphere impossible. It mandates the use of a vacuum or a constant flow of high-purity inert gas, such as argon, to protect the heating elements and insulation from rapid destruction.
Consumable Heating Elements
Even with a protective atmosphere, graphite elements have a finite lifespan. Over many high-temperature cycles, the graphite will slowly degrade or sublimate, becoming brittle and eventually failing.
This means the graphite furnace tube and insulation package are consumable parts that require periodic replacement, representing a significant ongoing operational cost.
Creation of a Carbon-Rich Atmosphere
At very high temperatures, graphite sublimates, releasing carbon atoms into the furnace's atmosphere. The references note this can be used intentionally for processes like carbonization or graphitization.
However, for many other applications, this is a major disadvantage. This carbon-rich environment can contaminate the material being processed, which is unacceptable for many metals, alloys, and ceramics where purity is critical.
Risk of Unwanted Carbide Formation
A direct consequence of the carbon-rich atmosphere is the potential for carbide formation.
When processing certain materials like refractory metals (tungsten, molybdenum) or some ceramics, the free carbon can react with the sample's surface. This forms an unintended carbide layer, altering the material's fundamental properties.
Understanding the Operational Trade-offs
The chemical reactivity of graphite creates several practical trade-offs that must be considered when choosing a furnace technology. While fast and powerful, it is not a universally applicable tool.
Requirement for Complex Atmospheres
The need for a vacuum or inert gas adds complexity and cost to the system. It requires vacuum pumps, gas delivery systems, and precise controls to prevent leaks. An air leak during a high-temperature run can lead to catastrophic failure of the hot zone.
Higher Long-Term Costs
While a graphite furnace may have a competitive initial purchase price, the total cost of ownership can be higher than alternatives.
Factoring in the recurring cost of replacement graphite elements, insulation, and the continuous consumption of expensive, high-purity inert gas is essential for a complete financial picture.
Limitations on Processable Materials
A graphite furnace is an unsuitable choice for any application where carbon is considered a contaminant. This immediately rules it out for many high-purity annealing, brazing, or sintering processes where maintaining the original chemistry of the material is the primary goal.
Making the Right Choice for Your Application
Selecting the right furnace depends entirely on balancing your process requirements against the inherent characteristics of the technology.
- If your primary focus is rapid heating for carbon-compatible materials: A graphite furnace is an excellent choice, as its speed can dramatically reduce cycle times.
- If your primary focus is processing oxygen-sensitive materials or avoiding carbon contamination: You must consider a furnace with an all-metal hot zone (using molybdenum or tungsten elements) to ensure a clean, carbon-free environment.
- If your primary focus is minimizing long-term operational costs: Carefully weigh the cost of consumable graphite and inert gas against the potentially higher initial investment of a more durable all-metal furnace.
Ultimately, understanding that a graphite furnace actively shapes its own atmosphere is the key to using it effectively or choosing a better alternative.
Summary Table:
| Key Disadvantage | Impact on Operation |
|---|---|
| Oxidation | Requires a vacuum or inert gas atmosphere, adding complexity and cost. |
| Carbon Contamination | Unsuitable for processes where material purity is critical. |
| Consumable Elements | Graphite parts degrade over time, leading to ongoing replacement costs. |
| Carbide Formation | Can alter material properties by forming unintended surface layers. |
Need a furnace that matches your material's purity requirements?
While powerful, graphite furnaces aren't the right fit for every application. The experts at KINTEK specialize in helping laboratories select the ideal heating solution—whether it's a graphite furnace for speed or an all-metal hot zone for ultimate purity.
We provide the right lab equipment and consumables to ensure your processes are efficient, cost-effective, and contamination-free.
Contact KINTEK today for a personalized consultation to find the perfect furnace for your specific materials and research goals.
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- Does graphite have a melting point? Unlocking the Extreme Heat Resistance of Graphite
- Why is graphite used in furnaces? For Extreme Heat, Purity, and Efficiency
- What are the applications of graphite material? Leveraging Extreme Heat and Precision for Industrial Processes



















