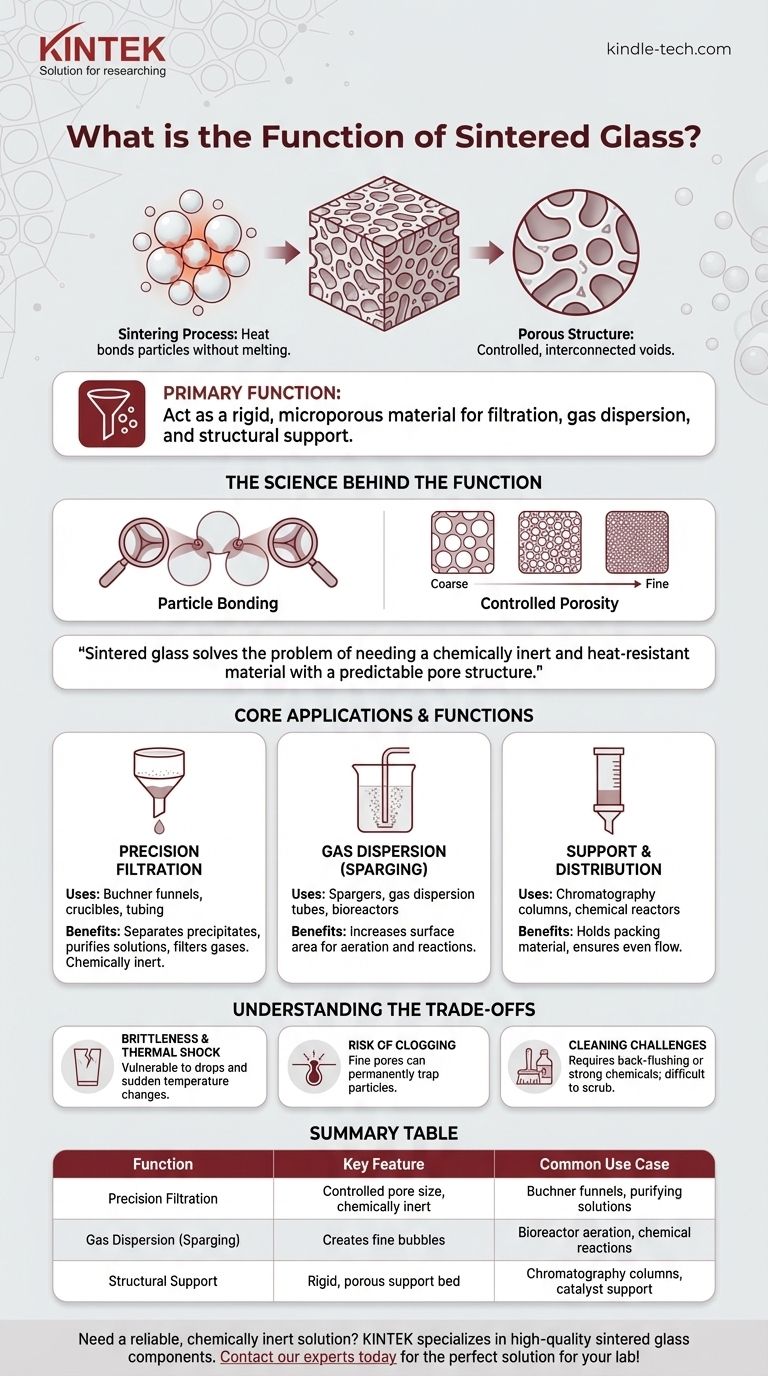
The primary function of sintered glass is to act as a rigid, microporous material for filtration, gas dispersion, and structural support. Unlike solid glass, it is created by heating glass powder until the particles fuse together, forming a solid mass riddled with precisely controlled, interconnected channels. This gives it the chemical resistance of glass combined with the functional properties of a fine filter.
Sintered glass solves the problem of needing a chemically inert and heat-resistant material with a predictable pore structure. Its function is not transparency, but rather to serve as a highly specialized filter or gas diffuser in scientific and industrial environments.

The Science Behind the Function
Sintered glass, often called fritted glass, derives its utility directly from its unique manufacturing process. Understanding this process is key to understanding its applications.
The Sintering Process
Sintering is a thermal process that bonds fine particles together into a solid piece without melting them into a liquid.
By heating glass powder to a temperature below its melting point, the surfaces of the individual glass grains become soft enough to weld together at their points of contact.
Creating a Porous Structure
This particle-welding process inherently leaves a network of tiny, interconnected voids between the fused grains.
The result is a single, rigid piece of glass that is porous. The size of the original glass particles directly controls the final pore size of the material, allowing for a high degree of manufacturing precision.
The Value of Controlled Porosity
This ability to create materials with specific pore sizes (from very coarse to ultra-fine) is the central reason sintered glass is so valuable.
It allows engineers and scientists to select a material that will block particles above a certain size while allowing fluids or gases to pass through freely.
Core Applications and Functions
The unique properties of sintered glass make it indispensable in settings where both chemical purity and physical separation are required.
Precision Filtration
The most common function of sintered glass is as a filter medium. It is integrated into laboratory glassware like Buchner funnels, crucibles, and tubing.
These filters are used to separate solid precipitates from a liquid, purify solutions, or filter particulate matter from gases. Because it is glass, it will not react with or contaminate the filtrate.
Gas Dispersion (Sparging)
Sintered glass tubes or discs, often called spargers or gas dispersion tubes, are used to bubble a gas through a liquid.
Forcing gas through the fine pores creates a stream of very small bubbles. This dramatically increases the surface area of contact between the gas and the liquid, which is critical for processes like aeration in bioreactors or efficient chemical reactions.
Support and Distribution
In chromatography columns or chemical reactors, a sintered glass disc can act as a support bed.
It holds the packing material (like a catalyst or resin) in place while allowing liquids or gases to flow through evenly across the entire column diameter.
Understanding the Trade-offs
While highly effective, sintered glass is not a universal solution. Its properties come with specific limitations that must be considered.
Brittleness and Thermal Shock
It is still glass. Sintered glass components are brittle and can be easily broken if dropped or subjected to mechanical stress.
Sudden, extreme temperature changes can also cause thermal shock, leading to cracks and failure.
Risk of Clogging
The very fine pores that make sintered glass an excellent filter also make it susceptible to permanent clogging.
If used to filter gelatinous substances or extremely fine particles that become lodged deep within the pores, it can be nearly impossible to clean and restore its original flow rate.
Cleaning Challenges
Cleaning requires care, often involving back-flushing with solvents or using strong chemical solutions like chromic acid, which carries its own safety hazards. Unlike a simple mesh screen, you cannot mechanically scrub the internal pores.
Making the Right Choice for Your Goal
Selecting sintered glass depends entirely on your specific requirements for chemical compatibility, purity, and particle size.
- If your primary focus is chemical inertness and fine particle filtration: Sintered glass is the superior choice for most laboratory and chemical processing applications where metal or polymer filters would react.
- If your primary focus is mechanical strength or high-pressure applications: You should consider sintered metal or ceramic filters, which offer far greater durability and resistance to physical shock.
- If your primary focus is dispersing gas efficiently into a chemically sensitive liquid: A sintered glass sparger is the ideal tool, providing fine bubbles without introducing contaminants.
Ultimately, understanding the function of sintered glass empowers you to select the precise tool needed to maintain purity and control in your scientific or industrial process.
Summary Table:
| Function | Key Feature | Common Use Case |
|---|---|---|
| Precision Filtration | Controlled pore size, chemically inert | Buchner funnels, crucibles, purifying solutions |
| Gas Dispersion (Sparging) | Creates fine bubbles for efficient gas-liquid contact | Bioreactor aeration, chemical reactions |
| Structural Support | Rigid, porous support bed | Chromatography columns, holding catalyst/resin |
Need a reliable, chemically inert filtration or gas dispersion solution for your lab?
KINTEK specializes in high-quality lab equipment and consumables, including sintered glass components designed for precision and purity. Our products ensure your processes maintain the highest standards of chemical resistance and performance.
Contact our experts today to find the perfect sintered glass solution for your specific laboratory needs!
Visual Guide
Related Products
- Optical Ultra-Clear Glass Sheet for Laboratory K9 B270 BK7
- 400-700nm Wavelength Anti Reflective AR Coating Glass
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- What is the use of hot isostatic pressing? Achieve Flawless Material Integrity for Demanding Applications
- What advantage does the electric arc furnace present in comparison to the basic oxygen furnace? Unlock Flexibility & Sustainability
- What is the cheapest type of additive manufacturing process? Start 3D Printing on a Budget with FDM
- Why graphite Cannot conduct electricity? Unlocking the Secret of its High Electrical Conductivity
- Why must a laboratory oven be used for the dehydration of sodium molybdate precursors? Ensure Synthesis Success
- What is the demand for synthetic diamonds? Rising Popularity for Ethical & Affordable Gems
- Why are vacuum pumps and pressure control systems necessary in an USP setup? Achieve High-Purity Powder Synthesis
- What role does a laboratory orbital shaker play in silane coupling? Enhance Self-Assembled Monolayer Uniformity

















