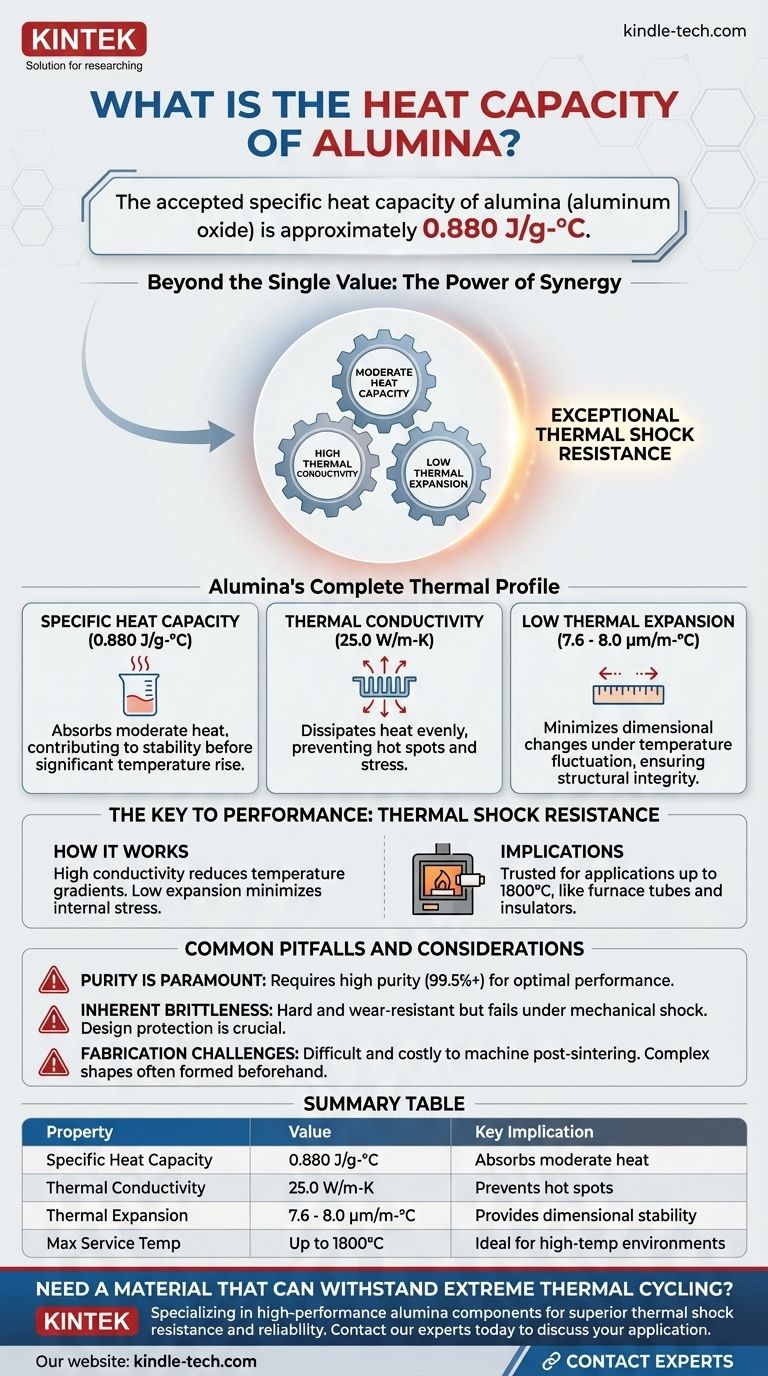
The accepted specific heat capacity of alumina (aluminum oxide) is approximately 0.880 J/g-°C. This value quantifies the amount of heat energy required to raise the temperature of one gram of the material by one degree Celsius. While this single data point is important, it only reveals part of the reason why alumina is a dominant material in high-temperature applications.
The true value of alumina lies not in any single thermal property, but in the powerful synergy between its moderate heat capacity, high thermal conductivity, and low thermal expansion. This combination is what grants it exceptional thermal shock resistance.

Understanding Alumina's Complete Thermal Profile
To properly evaluate alumina for any technical application, you must look beyond a single value and analyze how its key thermal properties work together.
The Role of Specific Heat Capacity
The specific heat capacity of 0.880 J/g-°C means alumina can absorb a moderate amount of heat energy before its temperature rises significantly.
This property contributes to its stability, but it is the interplay with other characteristics that defines its performance under thermal stress.
The Impact of Thermal Conductivity
Alumina possesses a relatively high thermal conductivity of 25.0 W/m-K.
This is a critical factor for high-temperature stability. It allows heat to dissipate quickly and evenly throughout the material, preventing the formation of localized hot spots that can induce stress and lead to failure.
The Advantage of Low Thermal Expansion
The coefficient of thermal expansion for alumina is very low, ranging from 7.6 to 8.0 µm/m-°C.
This means the material expands and contracts very little when subjected to large temperature changes. This dimensional stability is crucial for maintaining structural integrity and tight tolerances in components like furnace tubes and insulators.
The Key to Performance: Thermal Shock Resistance
The most significant outcome of these combined properties is alumina's excellent resistance to thermal shock—the ability to withstand rapid temperature changes without cracking.
How the Properties Work Together
The high thermal conductivity quickly reduces temperature gradients across the material, while the low thermal expansion minimizes the internal stress created by any remaining temperature differences.
This synergy allows alumina components to be heated or cooled rapidly, a requirement in many industrial and scientific processes.
Implications for High-Temperature Applications
This robust thermal profile is why alumina is a trusted material for applications operating up to 1800°C.
It is used for furnace linings, thermocouple protection tubes, and insulators in environments where other materials would fail due to thermal stress, chemical attack, or abrasion.
Common Pitfalls and Considerations
While alumina's thermal properties are outstanding, a complete technical assessment requires acknowledging its limitations.
Purity Is Paramount
The excellent properties cited here apply to high-purity alumina (typically 99.5% or higher). The presence of impurities, particularly silica, can dramatically reduce its maximum service temperature and overall performance.
Inherent Brittleness
Like most ceramics, alumina is hard and wear-resistant but also brittle. It has low fracture toughness and can fail catastrophically under mechanical shock or impact. Designs must protect it from tensile stress and direct impact.
Fabrication Challenges
The same hardness that provides excellent abrasion resistance makes alumina difficult and expensive to machine. Complex shapes are often formed before final sintering, as post-firing machining is a specialized and costly process.
Making the Right Choice for Your Application
To select the right material, you must align its properties with your primary engineering goal.
- If your primary focus is thermal stability and shock resistance: Alumina is an exceptional choice due to its unique combination of high conductivity and low expansion.
- If your primary focus is resisting mechanical impact or vibration: You must account for alumina's inherent brittleness in your design or consider tougher materials like zirconia.
- If your primary focus is creating complex components at low cost: Be aware that alumina's hardness makes post-sintering machining a significant cost driver, and plan accordingly.
Ultimately, understanding alumina's complete thermal and mechanical profile is the key to leveraging its exceptional high-temperature capabilities effectively.
Summary Table:
| Property | Value for High-Purity Alumina | Key Implication |
|---|---|---|
| Specific Heat Capacity | 0.880 J/g-°C | Absorbs moderate heat, contributing to thermal stability |
| Thermal Conductivity | 25.0 W/m-K | Prevents hot spots by dissipating heat evenly |
| Thermal Expansion Coefficient | 7.6 - 8.0 µm/m-°C | Provides exceptional dimensional stability under temperature changes |
| Maximum Service Temperature | Up to 1800°C | Ideal for demanding high-temperature environments |
Need a material that can withstand extreme thermal cycling?
At KINTEK, we specialize in high-performance lab equipment and consumables, including alumina components like furnace tubes and insulators. Our expertise ensures you get the right material solution for superior thermal shock resistance, durability, and long-term reliability in your laboratory processes.
Contact our experts today to discuss how high-purity alumina can solve your high-temperature application challenges.
Visual Guide
Related Products
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- High Purity Gold Platinum Copper Iron Metal Sheets
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Versatile PTFE Solutions for Semiconductor and Medical Wafer Processing
People Also Ask
- What is the main difference between soldering and brazing? Choose the Right Metal Joining Method
- What are the disadvantages of brazing? Understanding the key limitations and trade-offs.
- What is the function of a BN inner liner in a graphite mold during Flash Sintering? Master Precise Current Control
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- What are the strengths of brazing? Achieve Strong, Clean, and Precise Metal Joining















