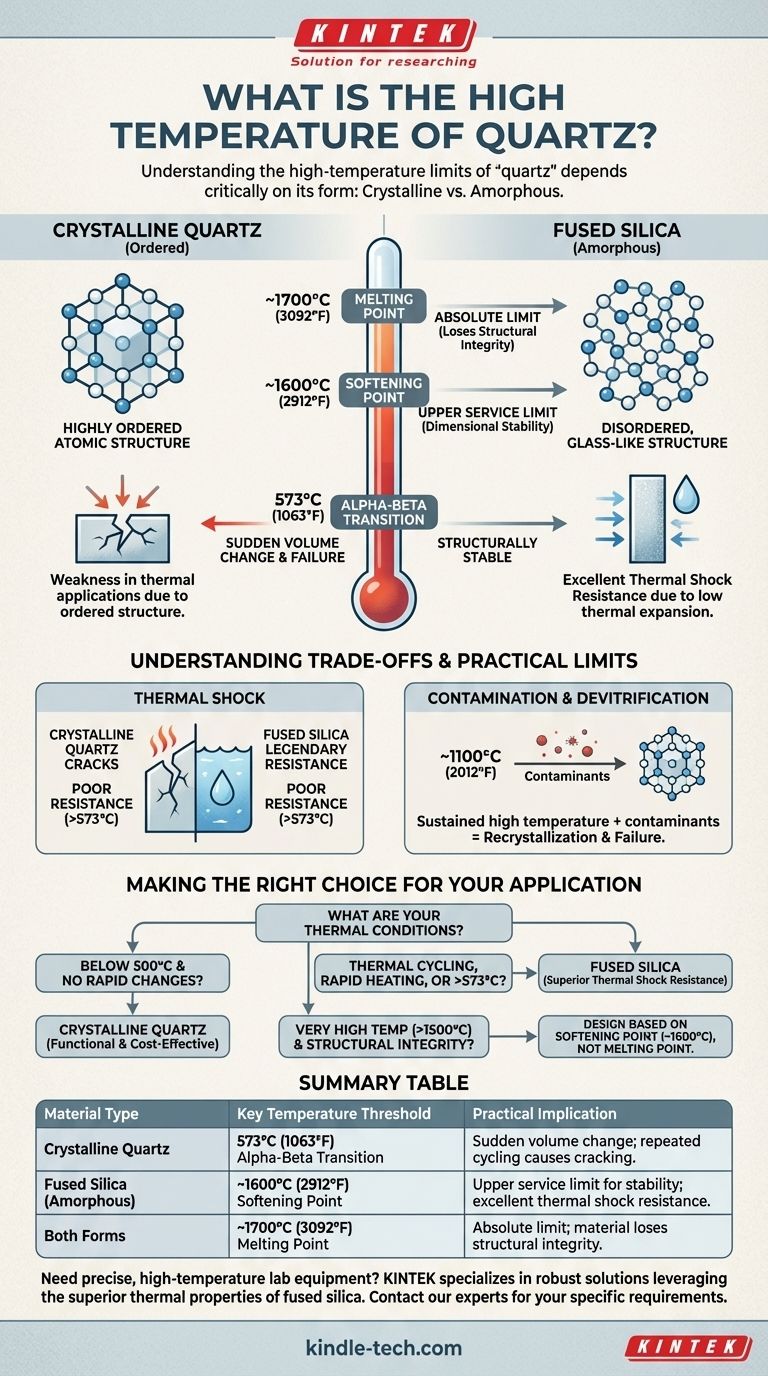
In practice, the high temperature of quartz depends critically on its form. While pure quartz melts at approximately 1700°C (3092°F), the most important temperature for natural crystalline quartz is its phase transition at 573°C (1063°F). For high-temperature applications requiring thermal stability, a non-crystalline form called fused silica is used, which has a much higher practical working limit near its softening point of 1600°C (2912°F).
Understanding the high-temperature limits of "quartz" is less about knowing a single melting point and more about distinguishing between its crystalline and amorphous forms. The practical temperature ceiling is defined by structural stability and resistance to thermal shock, not just melting.

The Critical Distinction: Crystalline Quartz vs. Fused Silica
Many professionals use the term "quartz" interchangeably, but in high-temperature environments, the difference between its two primary forms is the most important factor.
What is Crystalline Quartz?
Crystalline quartz is a mineral composed of silicon dioxide (SiO₂) in a highly ordered, repeating atomic structure. It is abundant in nature and is valued for its piezoelectric and optical properties.
However, its ordered structure is also its primary weakness in thermal applications.
What is Fused Silica?
Fused silica (often called fused quartz) is also made of pure silicon dioxide. The key difference is that it is amorphous, meaning its atomic structure is disordered and glass-like, not a rigid crystal lattice.
This material is manufactured by melting high-purity crystalline quartz and cooling it rapidly enough that crystals cannot re-form.
Why This Difference Matters for Temperature
The rigid, ordered structure of crystalline quartz undergoes a sudden change at a specific temperature. The disordered structure of fused silica does not.
This gives fused silica an extremely low coefficient of thermal expansion, making it exceptionally resistant to thermal shock.
Key Temperature Thresholds
The "high temperature" of quartz isn't one number, but a series of critical thresholds that dictate its practical use.
The 573°C Alpha-Beta Transition
This is the most critical temperature for crystalline quartz. At 573°C (1063°F), the material abruptly changes its crystal structure from alpha-quartz to beta-quartz.
This "quartz inversion" causes a sudden change in volume. Repeatedly heating and cooling crystalline quartz through this temperature will cause internal stress, leading to cracking and catastrophic failure.
The Softening Point (~1600°C)
This threshold is relevant for fused silica. The softening point is the temperature at which the material begins to lose its rigidity and will deform under its own weight.
For any application where dimensional stability is important, this is the true upper service limit, well below the actual melting point.
The Melting Point (~1700°C)
At approximately 1700°C (3092°F), both forms of quartz will fully melt into a viscous liquid.
While this is the absolute limit, it is rarely a useful number for design purposes, as the material loses all structural integrity long before this point.
Understanding the Trade-offs and Practical Limits
Choosing the right material requires understanding the risks associated with high-temperature use.
The Danger of Thermal Shock
Fused silica has legendary resistance to thermal shock. You can heat it to over 1000°C and plunge it into cold water without cracking. Its low thermal expansion allows it to tolerate extreme, rapid temperature changes.
Crystalline quartz, by contrast, has poor thermal shock resistance, especially when crossing its 573°C inversion point.
Contamination and Devitrification
Even fused silica can be compromised. At sustained temperatures above 1100°C (2012°F), contact with contaminants (like salts or metal oxides) can cause the amorphous structure to devitrify, or recrystallize.
This recrystallized area no longer has the thermal properties of fused silica and can become a point of mechanical failure.
Long-Term vs. Short-Term Exposure
The maximum service temperature is always dependent on time. A material might withstand a brief temperature spike but will deform or degrade if that same temperature is held for hours or days.
Making the Right Choice for Your Application
Your choice depends entirely on the thermal conditions of your project.
- If your primary focus is an application that stays below 500°C and avoids rapid temperature changes: Crystalline quartz can be a functional and cost-effective material.
- If your primary focus is thermal cycling, rapid heating, or any use above 573°C: Fused silica is the only reliable choice due to its superior thermal shock resistance.
- If your primary focus is structural integrity at very high temperatures (above 1500°C): You must design based on the softening point of fused silica, not its melting point, and account for potential material sag.
Ultimately, selecting the right material demands you look beyond a simple data sheet value and understand its true thermal behavior.
Summary Table:
| Material Type | Key Temperature Threshold | Practical Implication |
|---|---|---|
| Crystalline Quartz | 573°C (1063°F) Alpha-Beta Transition | Sudden volume change; repeated cycling causes cracking and failure. |
| Fused Silica (Amorphous) | ~1600°C (2912°F) Softening Point | Upper service limit for dimensional stability; excellent thermal shock resistance. |
| Both Forms | ~1700°C (3092°F) Melting Point | Absolute limit; material loses structural integrity long before this point. |
Need precise, high-temperature lab equipment? The right material choice is critical for your application's success and safety. KINTEK specializes in providing robust lab equipment and consumables, including solutions that leverage the superior thermal properties of fused silica. Our experts can help you select the perfect components to ensure thermal stability, resist shock, and extend the life of your labware. Contact our team today to discuss your specific high-temperature requirements and enhance your laboratory's capabilities.
Visual Guide
Related Products
- High Temperature Resistant Optical Quartz Glass Sheet
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- Zinc Selenide ZnSe Optical Window Glass Substrate Wafer and Lens
- 400-700nm Wavelength Anti Reflective AR Coating Glass
People Also Ask
- What temperature do you heat treat a furnace? It's All About Your Material and Goal
- What are the different types of physical vapour deposition processes? A Guide to Evaporation, Sputtering & More
- What are the limitations of sputtering process? Understand Key Trade-offs for Thin Film Deposition
- What is the function of a forced air drying oven in the regeneration cycle of dolomite catalysts? Optimize Your Lab Results
- What is an example of a continuous furnace? Discover the Conveyor Belt Furnace for High-Volume Production
- What is the recommended storage temperature for human serum? Preserve Sample Integrity for Reliable Results
- What are the advantages and disadvantages of a centrifuge? Weighing Speed Against Cost and Risk
- What are the factors affecting heat transfer efficiency? Optimize Your Thermal Management System



















