Potassium bromide (KBr) is a simple salt with a profoundly important dual identity in the worlds of science and medicine. Its primary modern significance lies in its role as an essential material for infrared (IR) spectroscopy, a cornerstone technique for chemical analysis. Simultaneously, it holds a historical and still-specialized place as a foundational anticonvulsant medication, particularly in veterinary practice.
The core importance of KBr stems from two distinct properties: its near-perfect transparency to infrared light, which makes it indispensable for analyzing chemical structures, and its physiological ability to suppress neurological overactivity, which established it as a pioneering treatment for epilepsy.

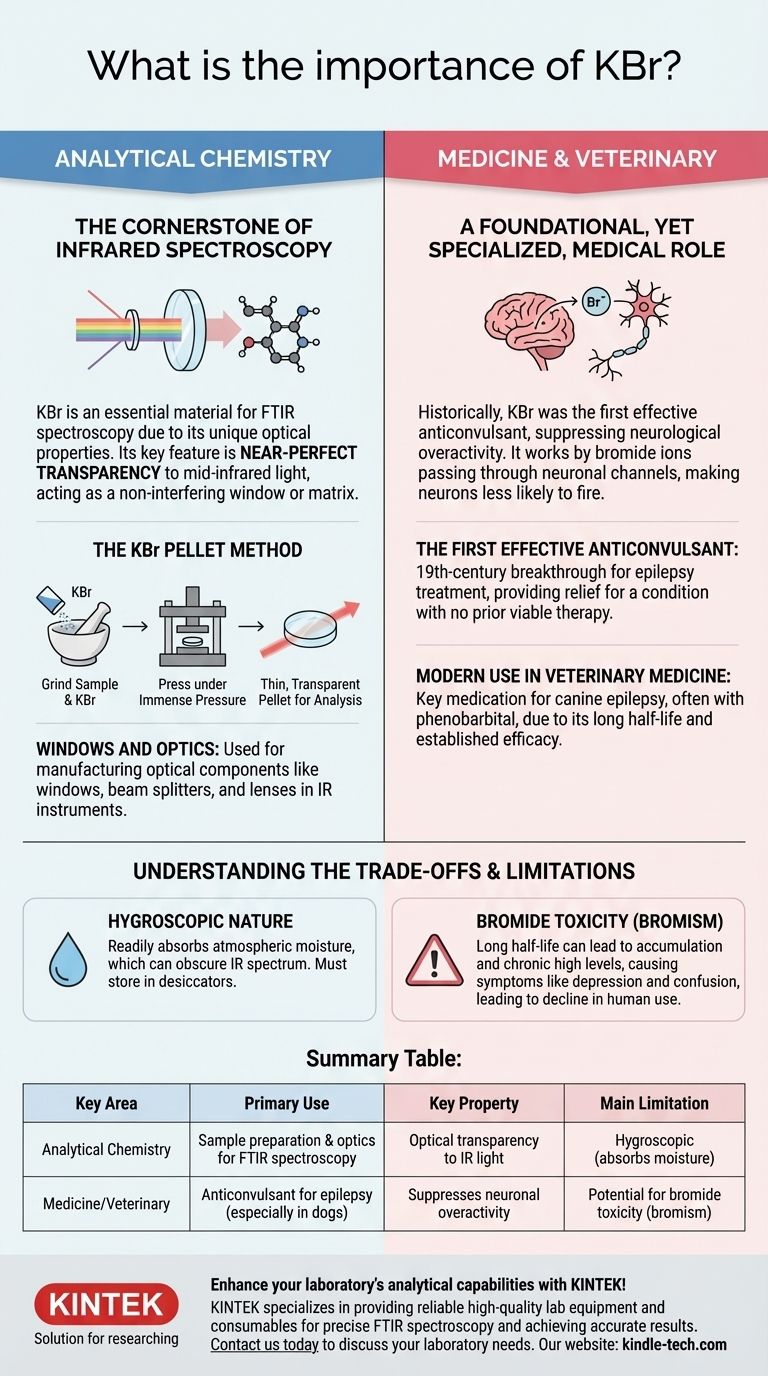
The Cornerstone of Infrared Spectroscopy
The most widespread and critical use of KBr today is in the field of analytical chemistry. Its unique optical properties make it an almost ideal medium for a powerful technique called Fourier-transform infrared (FTIR) spectroscopy.
Why Optical Transparency Matters
Most materials absorb specific frequencies of infrared light, which correspond to the vibrations of their chemical bonds. An FTIR instrument measures this absorption to identify a substance and its functional groups.
KBr is exceptional because it does not absorb light across a very wide range of the mid-infrared spectrum. This transparency means it can be used as a "window" or a matrix that doesn't interfere with the measurement of the actual sample being analyzed.
The KBr Pellet Method
For solid samples, the most common preparation technique involves KBr. The analyst grinds a tiny amount of the sample with a larger amount of high-purity KBr powder.
This mixture is then pressed under immense pressure in a die to form a thin, transparent pellet. When the IR beam passes through this pellet, the KBr remains invisible, and the resulting spectrum is purely that of the sample compound.
Windows and Optics
Beyond sample preparation, KBr's transparency makes it an excellent material for manufacturing the actual optical components—such as windows, beam splitters, and lenses—used inside IR spectrophotometers.
A Foundational, Yet Specialized, Medical Role
Before its widespread use in chemistry, KBr was famous for its medical applications. It was one of the first substances discovered to have a reliable effect on the central nervous system.
The First Effective Anticonvulsant
In the 19th century, KBr was introduced as the first effective treatment for epilepsy. Its sedative and anticonvulsant properties provided relief for a condition that previously had no viable therapy.
It works because the bromide ion can pass through neuronal channels that normally use the chloride ion, leading to a hyperpolarized state that makes neurons less likely to fire, thus suppressing seizure activity.
Modern Use in Veterinary Medicine
While largely replaced in human medicine by drugs with fewer side effects, KBr is still a key medication in veterinary practice.
It is frequently used, often in combination with phenobarbital, to control seizures in dogs with idiopathic epilepsy. Its long half-life and established efficacy make it a valuable tool for canine neurology.
Understanding the Trade-offs and Limitations
Despite its utility, KBr is not without its challenges. Its limitations are what led to its decline in human medicine and require careful handling in the lab.
Hygroscopic Nature
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. In spectroscopy, this is problematic because water has a strong IR absorption, which can obscure the sample's spectrum. KBr pellets and optics must be stored in desiccators.
Bromide Toxicity (Bromism)
The primary reason KBr fell out of favor for human use is its potential for toxicity. Because it has a very long half-life in the body, bromide ions can accumulate.
Chronic high levels lead to a condition called bromism, with symptoms including depression, confusion, psychosis, and a characteristic skin rash. This narrow therapeutic window prompted the search for safer alternatives.
How to Apply This Knowledge
Understanding KBr's dual role is key to appreciating its context in different professional fields.
- If your primary focus is chemical analysis: You will view KBr as the gold-standard, non-interfering matrix for preparing solid samples for IR spectroscopy.
- If your primary focus is veterinary medicine: You will recognize KBr as a viable and effective, though second-line, therapeutic agent for managing canine epilepsy.
- If your primary focus is pharmacology or medical history: KBr represents a critical case study in the evolution of therapeutics—a breakthrough discovery eventually superseded by safer, more targeted alternatives.
Ultimately, potassium bromide's importance is defined by its remarkable ability to be invisible to infrared light while having a very visible effect on the nervous system.
Summary Table:
| Key Area | Primary Use | Key Property | Main Limitation |
|---|---|---|---|
| Analytical Chemistry | Sample preparation & optics for FTIR spectroscopy | Optical transparency to IR light | Hygroscopic (absorbs moisture) |
| Medicine/Veterinary | Anticonvulsant for epilepsy (especially in dogs) | Suppresses neuronal overactivity | Potential for bromide toxicity (bromism) |
Enhance your laboratory's analytical capabilities with KINTEK!
Just as KBr is essential for precise FTIR spectroscopy, having the right high-quality lab equipment and consumables is fundamental to achieving accurate and reliable results. Whether you need spectroscopy supplies, sample preparation tools, or other laboratory essentials, KINTEK specializes in providing the reliable equipment your lab depends on.
Contact us today to discuss how our solutions can support your specific laboratory needs and drive your research forward.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- Why is my hydraulic press not holding pressure? Diagnose and Fix Common Leaks
- What is a wood pellet mill? Turn Waste Biomass into High-Density Fuel
- What is a press frame? The Foundation of Precision and Force Management in Presses
- How much does it cost to build a hydraulic press? A DIY Guide to Budgeting for Power and Safety
- Why are hydraulic presses required when studying FATT50? Precision Tools for Grain Refinement & Impact Toughness
- Where is press forging used? Manufacturing Large, High-Strength Metal Components
- Why is a laboratory hydraulic press required for ceramic target pre-forming? Enhance Density and Thin Film Quality
- Can hydraulics overheat? Prevent System Failure and Costly Downtime














