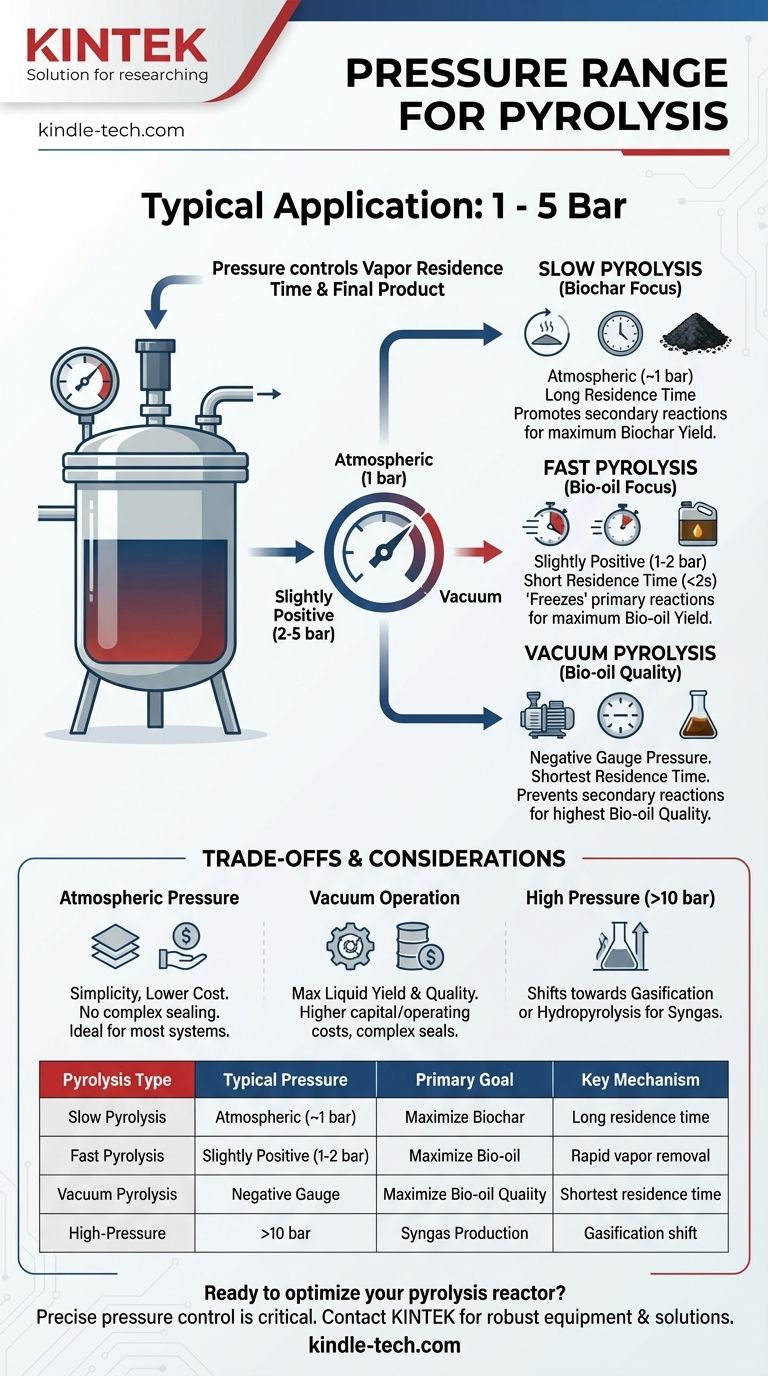
In most applications, pyrolysis is conducted at or near atmospheric pressure. While specialized high-pressure or vacuum conditions exist for specific outcomes, the vast majority of pyrolysis processes, from slow to fast, operate in a pressure range that is simple to engineer and manage, typically between 1 and 5 bar (atmospheric to slightly positive pressure).
The critical insight is that pressure in pyrolysis is not about reaching a specific high or low value; it's a tool used to control vapor residence time. This control is what ultimately determines whether the final output is predominantly biochar, bio-oil, or gas.

Why Pressure is a Critical Control Parameter
Pressure is one of the three key levers in pyrolysis, alongside temperature and heating rate. Its primary function is to influence how long the volatile gases created during the initial decomposition of biomass remain in the hot reaction zone.
The Role of Vapor Residence Time
Vapor residence time is the average duration that pyrolysis vapors spend within the reactor before they are condensed or removed.
A higher operating pressure forces these vapors to remain in the reactor for a longer period. Conversely, operating under a vacuum (negative pressure) or with a high flow of sweep gas pulls these vapors out almost instantly.
Impact on Product Yields
This residence time directly dictates the final product distribution.
Longer residence times (achieved at atmospheric or higher pressures) allow for secondary reactions, where the initial vapors break down further into non-condensable gases (like CO, H₂) and secondary char.
Shorter residence times (achieved with vacuum or rapid vapor removal) "freeze" the reaction at the primary decomposition stage. This preserves the condensable vapors, maximizing the yield of liquid bio-oil.
Pressure Conditions for Different Pyrolysis Types
The optimal pressure setting is entirely dependent on the desired end product.
Slow Pyrolysis (For Biochar)
Slow pyrolysis is typically performed at atmospheric pressure.
This condition, combined with slow heating rates, maximizes vapor residence time. It encourages the secondary reactions that crack vapors into more gas and, most importantly, deposit more carbon onto the solid fraction, maximizing the biochar yield.
Fast Pyrolysis (For Bio-oil)
Fast pyrolysis also operates near atmospheric pressure, often with a slight positive pressure (e.g., 1-2 bar).
While the pressure is atmospheric, the reactor is designed for extremely short vapor residence times (less than 2 seconds). The slight positive pressure helps to quickly push the vapors out of the reactor and into a quench system, preventing secondary reactions and maximizing the bio-oil yield.
Vacuum Pyrolysis (A Special Case for Bio-oil)
This method operates under a vacuum (negative gauge pressure).
By actively pulling vapors from the reaction zone, vacuum pyrolysis achieves the shortest possible residence time. This is the most effective way to prevent secondary reactions, often resulting in higher quality and quantity of bio-oil compared to atmospheric fast pyrolysis.
Understanding the Trade-offs
Choosing an operating pressure involves balancing process efficiency with engineering complexity and cost.
Operating at Atmospheric Pressure
The primary advantage is simplicity and lower cost. The equipment does not need to withstand significant pressure differentials, making reactor design and sealing much easier and more affordable. It is the default for most biochar and many bio-oil systems.
Operating Under Vacuum
The main benefit is maximum liquid yield and quality. The downside is significantly higher capital and operating costs. Vacuum systems require more complex reactor seals, robust construction, and powerful vacuum pumps, increasing both complexity and the risk of air leaking into the system.
Operating at High Pressure
Pressures significantly above atmospheric (e.g., >10 bar) fundamentally change the process, moving it toward gasification or hydropyrolysis. This is a different thermochemical regime used primarily for producing syngas or directly upgrading bio-oils in the presence of a catalyst and hydrogen.
Matching Pressure to Your Pyrolysis Goal
Your target output dictates the pressure strategy.
- If your primary focus is maximizing biochar yield: Use slow pyrolysis at standard atmospheric pressure to encourage secondary reactions.
- If your primary focus is maximizing bio-oil yield: Use fast pyrolysis near atmospheric pressure with rapid vapor quenching, or use vacuum pyrolysis for the highest possible quality and yield.
- If your primary focus is producing syngas: You are moving beyond typical pyrolysis into high-pressure gasification processes.
- If your primary focus is simplicity and low cost: Design your system to operate at atmospheric pressure, which is sufficient for producing both good-quality biochar and bio-oil.
Ultimately, pressure is the lever you pull to direct the chemical pathways inside the reactor and achieve your desired end product.
Summary Table:
| Pyrolysis Type | Typical Pressure Range | Primary Goal | Key Mechanism |
|---|---|---|---|
| Slow Pyrolysis | Atmospheric (~1 bar) | Maximize Biochar | Long vapor residence time for secondary reactions |
| Fast Pyrolysis | Slightly Positive (1-2 bar) | Maximize Bio-oil | Rapid vapor removal to 'freeze' primary reactions |
| Vacuum Pyrolysis | Negative Gauge Pressure | Maximize Bio-oil Quality | Shortest possible vapor residence time |
| High-Pressure | >10 bar | Syngas Production | Shifts process toward gasification/hydropyrolysis |
Ready to build or optimize your pyrolysis reactor for maximum yield? The precise control of pressure is critical for directing your product output. At KINTEK, we specialize in providing robust laboratory equipment and consumables designed for demanding thermochemical processes like pyrolysis. Whether you're developing a new bio-oil process or scaling up biochar production, our expertise can help you select the right components for your pressure and temperature requirements.
Contact our experts today to discuss how KINTEK's solutions can enhance your pyrolysis research and development.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the contribution of a hydrothermal reactor to graded pore construction? Precision Templates for TAS
- What is the function of a constant temperature hydrothermal reactor? Master Coal Fly Ash Activation
- What is the purpose of using a high-temperature hydrothermal reactor? Enhance Iodine@Activated Carbon Cathode Synthesis
- What role does an autoclave play in simulating PWR conditions? Advanced Material Validation for Nuclear Safety
- Why are high-pressure autoclaves essential for preparing bio-based polyamide curing agents from dimeric acid?



















