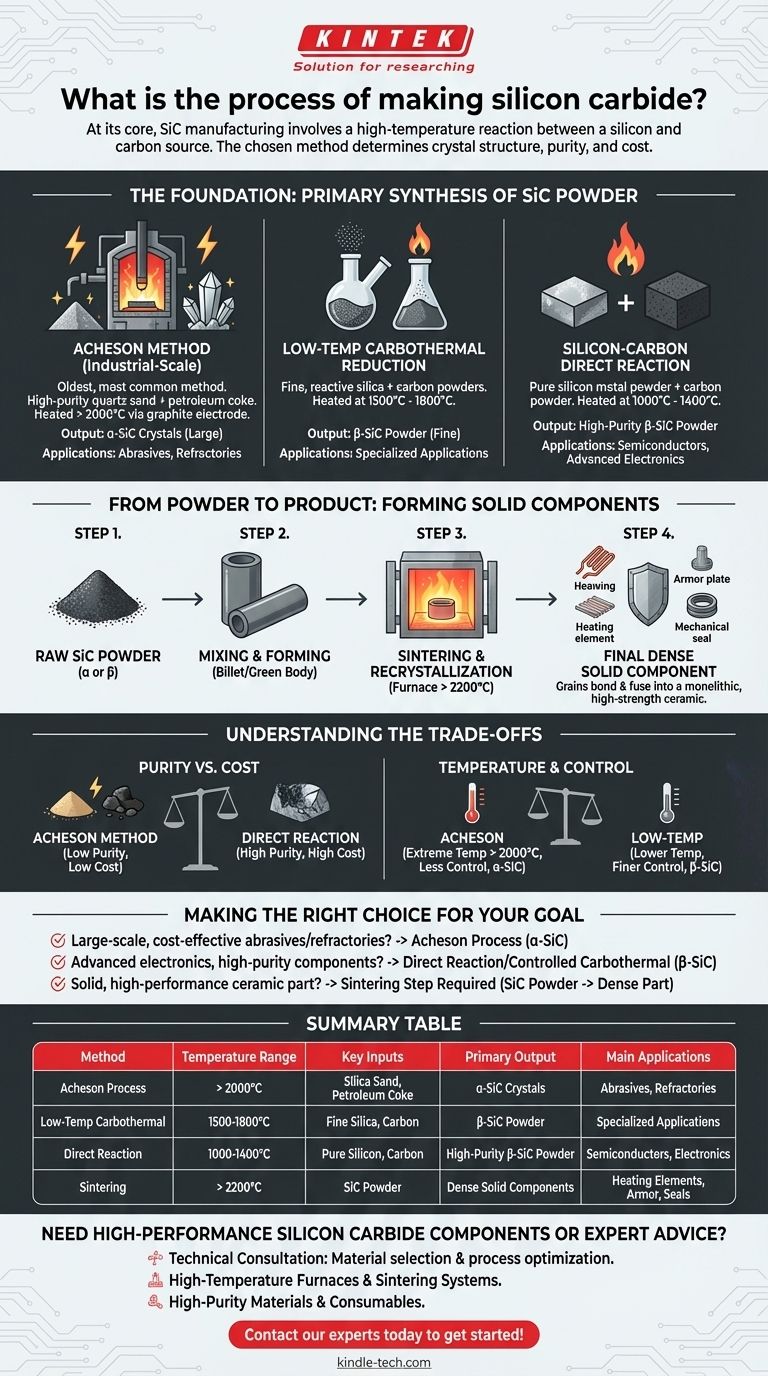
At its core, the manufacturing of silicon carbide (SiC) involves a high-temperature chemical reaction between a silicon source and a carbon source. The most common industrial method, known as the Acheson process, heats a mixture of silica sand (silicon dioxide) and petroleum coke (carbon) in a large resistance furnace to temperatures exceeding 2000°C, causing the materials to react and form silicon carbide crystals.
The specific manufacturing method chosen is not arbitrary; it directly determines the crystal structure, purity, and cost of the final silicon carbide, tailoring it for applications ranging from simple abrasives to advanced electronic components.

The Foundation: Primary Synthesis of SiC Powder
The initial creation of silicon carbide always begins by synthesizing it into a powder or crystalline mass. There are three primary industrial methods, each with distinct parameters and outcomes.
The Acheson Method: Industrial-Scale Production
This is the oldest and most common method for bulk SiC production. A massive furnace is loaded with a mixture of high-purity quartz sand and finely ground petroleum coke.
An electric current is passed through a graphite core, generating immense heat (above 2000°C). This carbothermal reduction synthesizes large crystals of alpha-silicon carbide (α-SiC) over several days.
Low-Temperature Carbothermal Reduction
This method offers more control by reacting finer, more reactive silica and carbon powders at lower temperatures, typically between 1500°C and 1800°C.
The result is a fine powder of beta-silicon carbide (β-SiC), a different crystal structure often desired for more specialized applications.
Silicon-Carbon Direct Reaction
For applications demanding the highest purity, this method reacts pure silicon metal powder directly with carbon powder.
This process runs at even lower temperatures (1000°C to 1400°C) and avoids the impurities inherent in sand and coke, yielding very high-purity β-SiC powder.
From Powder to Product: Forming Solid Components
Raw SiC powder or crystal is often just the starting point. To create durable products like heating elements, armor, or mechanical seals, the powder must be consolidated into a dense, solid form.
The Goal: Densification and Bonding
The objective of this secondary process is to fuse the individual silicon carbide grains together, eliminating the empty space between them and forming a monolithic ceramic part.
The Process: Sintering and Recrystallization
The SiC powder is first mixed with binding agents and processed into a preliminary shape, often called a "billet" or "green body."
This shape is then fired in a furnace at extremely high temperatures, often exceeding 2200°C. At this temperature, the grains bond and recrystallize, fusing into a solid, high-strength ceramic with excellent thermal and electrical properties.
Understanding the Trade-offs
The choice of manufacturing process involves a critical balance between cost, purity, and the final material properties.
Purity vs. Cost
The Acheson method is cost-effective for producing large quantities, making it ideal for abrasives like sandpaper. However, its use of raw sand and coke introduces impurities.
Conversely, the direct reaction method uses expensive, pre-purified silicon metal as a starting material, significantly increasing the cost but delivering the high purity needed for semiconductors and advanced electronics.
Temperature and Control
The extreme temperatures of the Acheson process are energy-intensive and result in the formation of α-SiC, the most stable crystal form.
Lower-temperature methods that produce β-SiC allow for finer control over particle size and purity but are generally more complex and less suited for massive bulk production.
Making the Right Choice for Your Goal
Understanding the different production pathways is key to selecting the correct type of silicon carbide for a specific engineering challenge.
- If your primary focus is large-scale, cost-effective abrasives or refractories: The Acheson process for producing bulk α-SiC is the industry standard.
- If your primary focus is advanced electronics or specialized components requiring high purity: A direct reaction or controlled carbothermal process to create β-SiC powder is the necessary path.
- If your primary focus is creating a solid, high-performance ceramic part: Your process will start with SiC powder and require a secondary forming and high-temperature sintering step to achieve final density.
Mastering the synthesis of silicon carbide is what transforms simple sand and carbon into one of the most versatile advanced materials available.
Summary Table:
| Method | Temperature Range | Key Inputs | Primary Output | Main Applications |
|---|---|---|---|---|
| Acheson Process | > 2000°C | Silica Sand, Petroleum Coke | α-SiC Crystals | Abrasives, Refractories |
| Low-Temp Carbothermal | 1500-1800°C | Fine Silica, Carbon | β-SiC Powder | Specialized Applications |
| Direct Reaction | 1000-1400°C | Pure Silicon, Carbon | High-Purity β-SiC Powder | Semiconductors, Electronics |
| Sintering | > 2200°C | SiC Powder | Dense Solid Components | Heating Elements, Armor, Seals |
Need High-Performance Silicon Carbide Components or Expert Advice?
KINTEK specializes in advanced materials and high-temperature processing solutions for laboratories and industry. Whether you are developing semiconductor components, specialized ceramics, or require custom sintering services, our expertise in lab equipment and consumables can help you achieve superior results.
We provide:
- Technical Consultation on material selection and process optimization.
- High-Temperature Furnaces and sintering systems tailored for SiC and other advanced ceramics.
- High-Purity Materials and consumables to meet your specific research and production needs.
Let's discuss how we can support your project. Contact our experts today to get started!
Visual Guide
Related Products
- Mesh belt controlled atmosphere furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
People Also Ask
- Why is precise temperature control in a sintering furnace critical for NASICON electrolytes? Ensure Material Purity
- What is the role of an atmosphere-controlled tube furnace in Cu-Mo sintering? Achieve High-Purity Densification
- What is the core function of a high-temperature atmosphere sintering furnace in the fabrication of Ni-Al2O3-TiO2 composites?
- Why is a high-precision atmosphere furnace essential for high-nickel cathode sintering? Unlock Battery Performance
- Why Use Ultra-High Vacuum Furnaces for LLZO? Ensure Chemical Stability & Interface Integrity in Solid Electrolytes



















