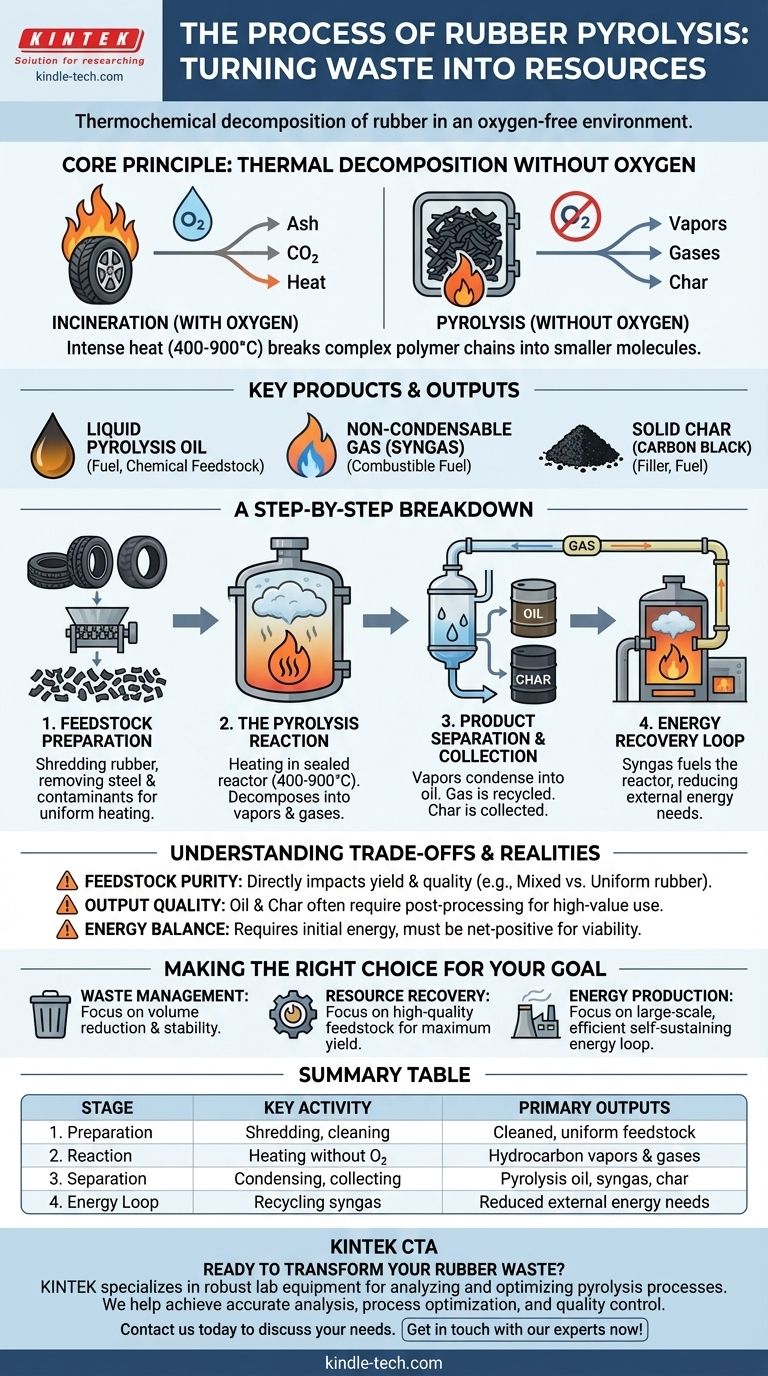
At its core, rubber pyrolysis is a thermochemical decomposition process that breaks down waste rubber using high heat in an oxygen-free environment. Instead of burning the material, this controlled process deconstructs its complex polymer chains, converting a problematic waste stream into valuable outputs: a liquid fuel similar to oil, a combustible gas, and a solid carbon-rich char.
Pyrolysis should be understood not as waste disposal, but as resource transformation. It chemically recycles waste rubber back into raw materials, but the economic and environmental viability of the process hinges on the purity of the feedstock and the control of process conditions.

The Core Principle: Thermal Decomposition Without Oxygen
Pyrolysis works by fundamentally altering the chemical structure of rubber through intense heat in a controlled, inert atmosphere.
What Happens Inside the Reactor?
The shredded rubber material is fed into a sealed reactor and heated to temperatures between 400°C and 900°C. This extreme heat, without the presence of oxygen, causes the long polymer chains that make up the rubber to vibrate violently and break apart into smaller, less complex molecules.
Why No Oxygen?
The absence of oxygen is the defining characteristic of pyrolysis and is what separates it from incineration. With oxygen, the material would simply burn (combust), producing mostly ash, carbon dioxide, and heat. By removing oxygen, we prevent combustion and instead force the material to thermally decompose into its constituent components.
The Key Products
This decomposition results in three primary outputs:
- Vapors, which are later condensed into a liquid pyrolysis oil.
- Non-condensable gases (syngas), which are combustible.
- A solid residue, which is a form of carbon black or char.
A Step-by-Step Breakdown of the Process
A commercial rubber pyrolysis operation follows a logical sequence from waste intake to final product storage.
Step 1: Feedstock Preparation
Raw waste rubber, such as old tires or industrial scraps, is first processed. This typically involves shredding the material into smaller, uniform pieces to ensure even heating. Critically, non-rubber contaminants like steel wires (in tires), fibers, and dirt are removed to prevent contamination of the final products.
Step 2: The Pyrolysis Reaction
The prepared rubber is fed into the pyrolysis reactor. The system is sealed to create an oxygen-free (anaerobic) environment, and heat is applied. As the material decomposes, it releases a mixture of hydrocarbon vapors and gases.
Step 3: Product Separation and Collection
This hot gas mixture exits the reactor and enters a separation and cooling system.
- Condensation: The vapors are passed through condensers, where they cool and turn into liquid pyrolysis oil, which is collected in storage tanks.
- Gas Recycling: The remaining non-condensable gases (syngas) are redirected.
- Solid Discharge: The solid char is removed from the bottom of the reactor, cooled, and collected.
Step 4: Energy Recovery Loop
A key feature of efficient pyrolysis plants is the use of the captured syngas. This combustible gas is often piped back to the furnace that heats the reactor, providing a significant portion of the energy required for the process. This creates a self-sustaining energy loop and reduces external fuel costs.
Understanding the Trade-offs and Realities
While promising, rubber pyrolysis is not a magic bullet. Its success depends on navigating several practical challenges.
Feedstock Purity is Paramount
The quality and consistency of the incoming rubber waste directly impact the yield and quality of the outputs. Mixed materials, such as a blend of different rubber types or contaminants, will produce lower-grade oil and char. For example, oil yields can range from as low as 20% for mixed sneakers to a more viable 35% for uniform rubber cables.
Output Quality and Post-Processing
The raw pyrolysis oil is not a direct replacement for refined diesel. It often requires further processing, such as distillation or purification, to be used as a stable fuel in engines or as a chemical feedstock. Likewise, the quality of the carbon black determines whether it can be sold as a high-value product or is only suitable for use as a low-grade solid fuel.
Energy Balance is Crucial
While recycling syngas makes the process more efficient, the system still requires a significant initial energy input to reach operating temperature. A successful operation must be designed to be net-energy positive, where the value of the outputs and the energy saved by the syngas loop outweigh the initial energy and operational costs.
Making the Right Choice for Your Goal
The application of pyrolysis technology should be aligned with a clear objective.
- If your primary focus is waste management: Pyrolysis is an excellent method for dramatically reducing the volume of non-biodegradable rubber waste and converting it into stable, storable, and potentially valuable materials.
- If your primary focus is resource recovery: Success depends entirely on securing a consistent, clean feedstock to maximize the yield of valuable pyrolysis oil and high-grade carbon char.
- If your primary focus is energy production: The process can be largely self-sustaining on a large scale, but its viability as a net power source requires careful engineering to ensure high efficiency and minimal energy loss.
Ultimately, rubber pyrolysis offers a powerful solution for turning a persistent environmental problem into a source of valuable resources.
Summary Table:
| Stage | Key Activity | Primary Outputs |
|---|---|---|
| 1. Preparation | Shredding rubber, removing contaminants | Cleaned, uniform feedstock |
| 2. Reaction | Heating in an oxygen-free reactor (400-900°C) | Hydrocarbon vapors and gases |
| 3. Separation | Condensing vapors, collecting solids | Pyrolysis oil, syngas, carbon char |
| 4. Energy Loop | Recycling syngas to fuel the reactor | Reduced external energy needs |
Ready to Transform Your Rubber Waste into Valuable Resources?
KINTEK specializes in providing robust lab equipment and consumables for analyzing and optimizing pyrolysis processes. Whether you are a researcher developing new methods or an industrial operation scaling up, our precise tools help you maximize yield and quality from your rubber feedstock.
We help our laboratory customers achieve:
- Accurate Analysis: Precisely monitor pyrolysis conditions and output quality.
- Process Optimization: Fine-tune temperature and feedstock parameters for better efficiency.
- Quality Control: Ensure the purity and value of your final oil, gas, and char products.
Contact us today to discuss how our solutions can support your pyrolysis research or operation. Let's turn your waste management challenge into a resource recovery success.
Get in touch with our experts now!
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Lab Internal Rubber Mixer Rubber Kneader Machine for Mixing and Kneading
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the principle of rotary kiln? Mastering Continuous Thermal Processing
- What are the industrial applications of pyrolysis? Transform Waste into Energy and Valuable Products
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker
- What are the products of pyrolysis of wood? A Guide to Biochar, Bio-oil, and Syngas Yields
- What equipment is used in pyrolysis? Choosing the Right Reactor for Your Feedstock and Products



















