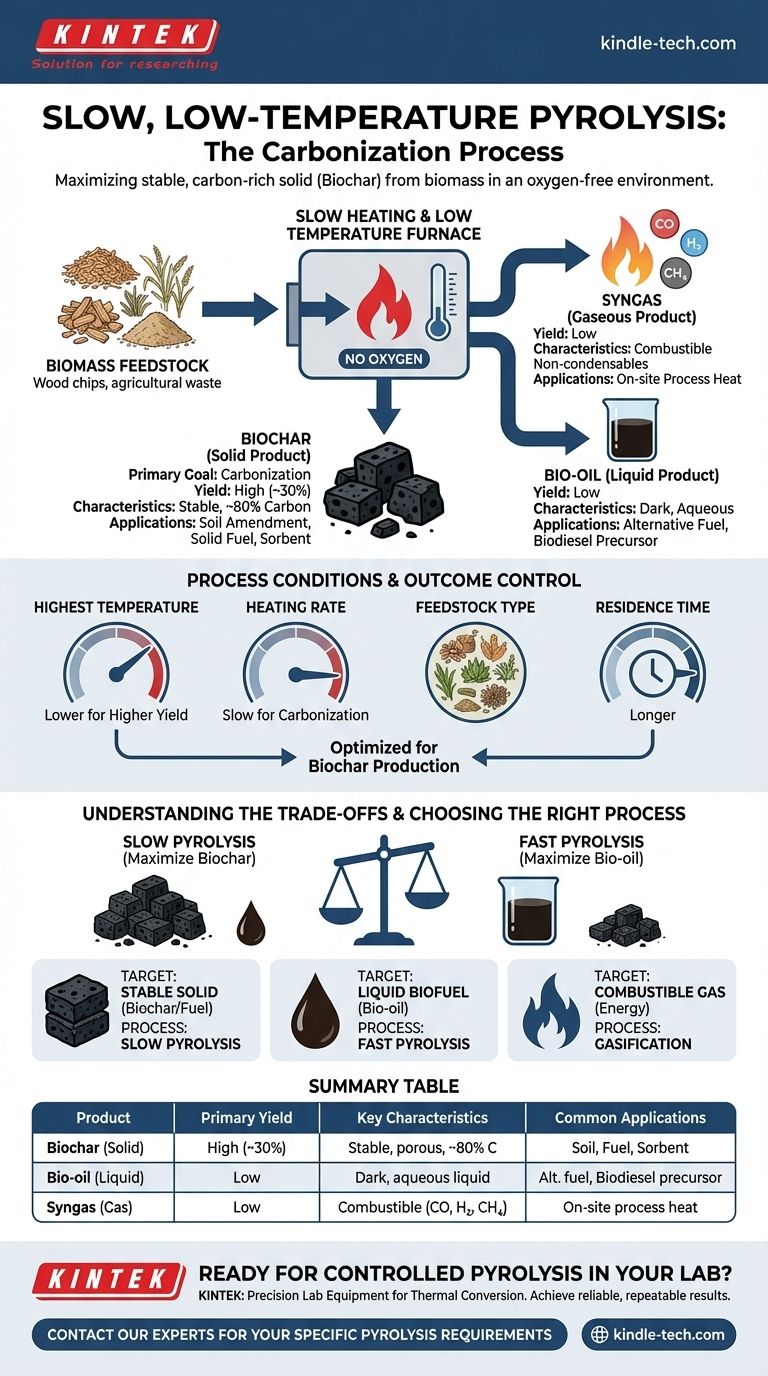
Slow, low-temperature pyrolysis is a thermal conversion process designed to maximize the production of a stable, carbon-rich solid. Also known as carbonization, this method involves heating organic material like biomass in an environment with no oxygen. This process yields three core products: a solid known as biochar (or charcoal), a liquid called bio-oil (or wood vinegar), and a non-condensable syngas.
The central purpose of slow pyrolysis is fundamentally different from other thermal processes. It is best understood as carbonization—a deliberate method where process conditions are optimized to convert organic matter into a high-yield, stable solid product (biochar), rather than liquid fuel.

The Three Primary Products of Slow Pyrolysis
Slow pyrolysis carefully breaks down organic material, separating it into distinct solid, liquid, and gaseous components. The yields and properties of each are highly dependent on the starting material and the precise process conditions.
The Solid Product: Biochar
This is the principal and most emphasized product of slow pyrolysis. It is a stable, black, and highly porous material consisting of around 80% carbon.
Under typical slow pyrolysis conditions, biochar yields can be as high as 30% of the initial dry feedstock weight. Its primary applications include agriculture (as a soil amendment), energy (as briquettes), and environmental remediation (as a sorbent).
The Liquid Product: Bio-oil
As the organic material heats up, volatile compounds evaporate and are collected as they condense back into a liquid. This product is commonly known as bio-oil, pyrolysis oil, or wood vinegar.
This dark, aqueous liquid can be used as an alternative fuel source or, with further refining, can be upgraded into products like biodiesel.
The Gaseous Product: Syngas
This fraction consists of non-condensable gases, including carbon monoxide, hydrogen, and methane. This pyrolysis gas is highly combustible.
In most modern pyrolysis plants, this syngas is not wasted. It is captured and consumed on-site to generate the heat required to sustain the pyrolysis reaction, making the process more energy-efficient.
How Process Conditions Dictate the Outcome
You cannot simply heat a material and expect a consistent result. The final output is a direct consequence of several carefully controlled variables. The goal is to give the volatile compounds time to evolve, leaving behind a stable carbon structure.
The Dominance of Temperature
Among all process factors, the highest treatment temperature has the most significant influence on the final characteristics of the biochar. Higher temperatures generally lead to a more refined, higher-carbon biochar but can reduce the overall solid yield.
The Role of Heating Rate
Slow pyrolysis is defined by its slow heating rate. This allows for a more complete carbonization process, maximizing the conversion of the biomass into the solid char structure, as opposed to fast pyrolysis which uses rapid heating to favor liquid bio-oil production.
Feedstock and Residence Time
The type of organic material used (feedstock) and the duration it is held at the peak temperature (residence time) are also critical. These factors, along with the specific gas environment and pressure, directly influence the final yield and chemical properties of all three products.
Understanding the Trade-offs
Choosing a thermal conversion process involves balancing competing priorities. Slow pyrolysis is optimized for one outcome, which inherently limits its efficiency in achieving others.
The Biochar vs. Bio-oil Compromise
The most fundamental trade-off is between solid and liquid yields. Slow pyrolysis is deliberately designed to maximize biochar at the expense of bio-oil. If your goal is to create a liquid fuel, this process is inefficient by design.
Process Control vs. Product Variability
While the process parameters can be tightly controlled, the final products remain highly dependent on the initial feedstock. The variability in biomass (e.g., wood chips vs. agricultural waste) will result in biochar and bio-oil with different properties, making it difficult to establish a standardized market price.
Making the Right Choice for Your Goal
Selecting the correct thermal process depends entirely on your desired end product.
- If your primary focus is creating a stable soil amendment or solid fuel: Slow pyrolysis is the correct process, as its goal is to maximize the yield of solid biochar.
- If your primary focus is producing liquid biofuel (bio-oil): You should investigate fast pyrolysis, a different technique that uses rapid heating to optimize liquid yields instead of solid char.
- If your primary focus is maximizing combustible gas for energy: Gasification, a related process that uses a small amount of oxygen, would be a more direct and efficient method.
Ultimately, understanding that slow pyrolysis is fundamentally a method of carbonization is the key to leveraging it effectively for your specific application.
Summary Table:
| Product | Primary Yield | Key Characteristics | Common Applications |
|---|---|---|---|
| Biochar (Solid) | High (~30%) | Stable, porous, ~80% carbon | Soil amendment, solid fuel, sorbent |
| Bio-oil (Liquid) | Low | Dark, aqueous liquid | Alternative fuel, biodiesel precursor |
| Syngas (Gas) | Low | Combustible (CO, H₂, CH₄) | On-site process heat |
Ready to implement a controlled pyrolysis process in your lab?
KINTEK specializes in precision lab equipment for thermal conversion processes like pyrolysis. Whether you are researching biochar for carbon sequestration, analyzing bio-oils, or developing new biomass applications, our reactors and furnaces provide the precise temperature control and process consistency you need.
We serve laboratories and research institutions focused on sustainable materials and energy. Let us help you achieve reliable, repeatable results.
Contact our experts today to discuss your specific pyrolysis requirements and find the ideal solution for your research goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Bomb Type Probe for Steelmaking Production Process
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















