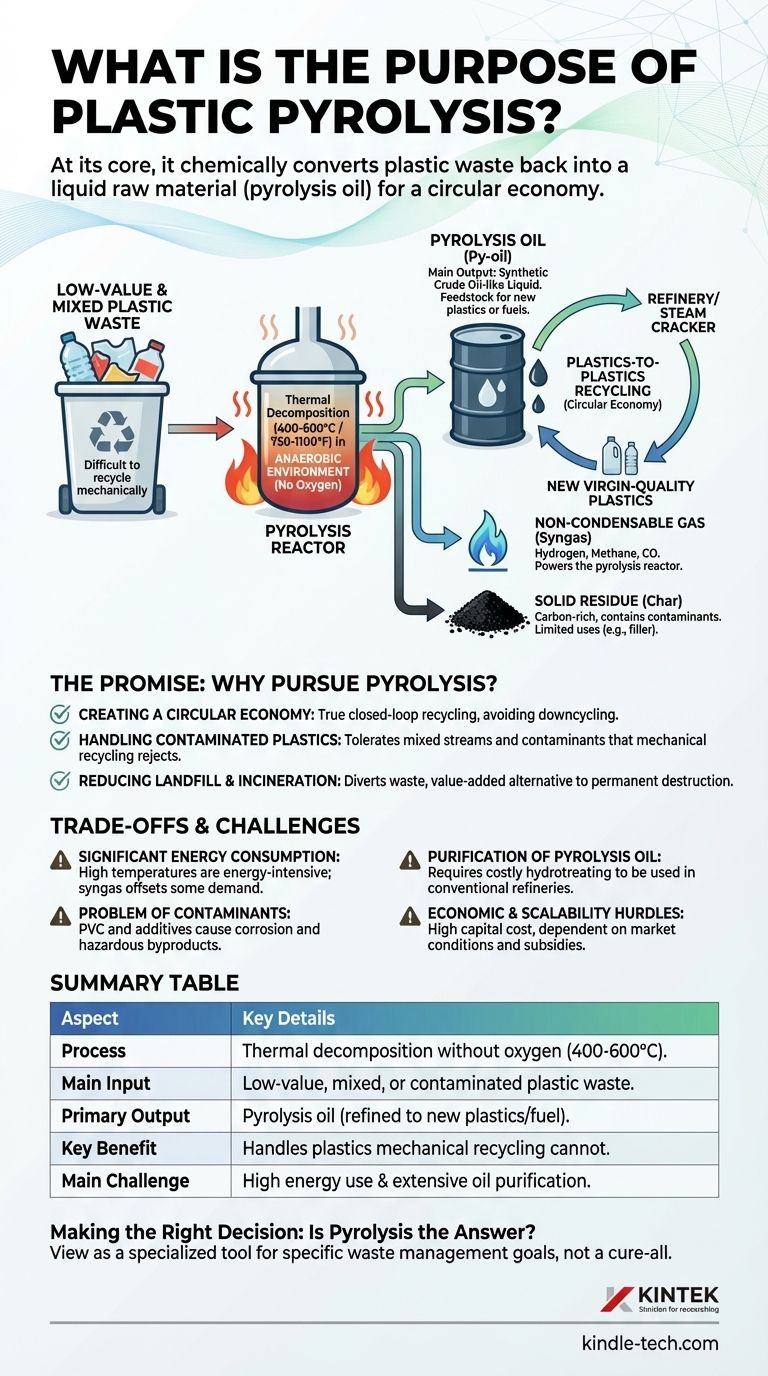
At its core, the purpose of plastic pyrolysis is to chemically convert plastic waste back into a liquid raw material, often called pyrolysis oil. This process uses high heat in the complete absence of oxygen to break down long plastic polymer chains into smaller, simpler hydrocarbon molecules. It is a form of advanced or chemical recycling designed to tackle plastics that are difficult or impossible to recycle mechanically.
While conventional recycling melts and remolds plastic, often degrading its quality, pyrolysis aims to deconstruct it at a molecular level. This creates a feedstock for new plastics or fuels, offering a potential pathway to a true circular economy for a material that would otherwise end up in a landfill or incinerator.

How Plastic Pyrolysis Works: The Core Process
To understand the purpose of pyrolysis, you must first understand the fundamental mechanism. It is a thermochemical process, distinct from both simple melting and outright burning (incineration).
The Feedstock: Beyond the Recycling Bin
Pyrolysis is particularly valuable for its ability to handle low-value and mixed plastic waste. This includes materials that traditional recycling facilities reject, such as flexible films, multi-layered food packaging, and contaminated containers. These are the most challenging components of the plastic waste stream.
The Reaction: Thermal Decomposition Without Oxygen
The sorted plastic is shredded and fed into a reactor. It is then heated to temperatures between 400°C and 600°C (750°F to 1100°F) in an anaerobic (oxygen-free) environment. The absence of oxygen is critical; it prevents the plastic from combusting and ensures it breaks down into its constituent hydrocarbon parts instead of turning into ash and flue gas.
The Outputs: Oil, Gas, and Char
The process yields three primary products:
- Pyrolysis Oil (Py-oil): This is the main output, a synthetic crude oil-like liquid. It's a complex mixture of hydrocarbons that can be upgraded and refined.
- Non-Condensable Gas (Syngas): A mixture of hydrogen, methane, carbon monoxide, and other light gases. This is almost always captured and used to power the pyrolysis reactor itself, reducing the process's reliance on external energy.
- Solid Residue (Char): A carbon-rich solid, similar to charcoal. It contains contaminants and fillers from the original plastic. Its uses are limited, but research is exploring its potential as a filler or in filtration.
The Promise: Why Pursue Pyrolysis?
The push for pyrolysis technology is driven by the severe limitations of our current waste management systems. It offers several potential advantages.
Creating a Circular Economy
The ultimate goal of pyrolysis is "plastics-to-plastics" recycling. The pyrolysis oil, after significant purification, can be fed into a refinery or steam cracker to produce the building blocks (like ethylene and propylene) for making new, "virgin-quality" plastics. This closes the loop in a way that mechanical recycling, which often results in downcycling, cannot.
Handling Contaminated and Mixed Plastics
Mechanical recycling requires clean, well-sorted streams of a single type of plastic (e.g., PET bottles). Pyrolysis is far more tolerant of the contamination and mixed streams that constitute the majority of post-consumer plastic waste, diverting it from landfills.
Reducing Landfill and Incineration Dependence
By providing a value-added pathway for non-recyclable plastic, pyrolysis directly reduces the volume of waste sent to landfills. It is also positioned as a more environmentally sophisticated alternative to incineration, which recovers energy but permanently destroys the material resource.
Understanding the Trade-offs and Challenges
As with any industrial process, pyrolysis is not a silver bullet. An objective assessment requires acknowledging its significant hurdles.
Significant Energy Consumption
Heating large volumes of material to high temperatures is an energy-intensive process. While the syngas produced can offset some of this demand, the net energy balance of a pyrolysis facility is a critical factor in its overall environmental and economic viability.
The Problem of Contaminants
Certain plastics create major problems. Polyvinyl chloride (PVC), for instance, releases chlorine, which forms highly corrosive hydrochloric acid in the reactor. Other additives, flame retardants, and pigments can end up in the oil or char, complicating their end use and potentially creating hazardous byproducts.
Purification of Pyrolysis Oil
The raw pyrolysis oil is not a "drop-in" replacement for fossil crude. It is often acidic, unstable, and contains oxygen, nitrogen, and other heteroatoms. It requires an extensive and costly upgrading process (hydrotreating) before it can be used in a conventional refinery. This purification step is one of the biggest economic and technical challenges facing the industry.
Economic and Scalability Hurdles
The high capital cost of building a pyrolysis plant, combined with the operational expense of energy and oil purification, makes the economics challenging. The process is often only viable with government subsidies or when oil prices are high, making it difficult to scale globally without favorable market conditions.
Making the Right Decision: Is Pyrolysis the Answer?
Pyrolysis is best understood as a specific tool for a specific problem within the broader plastic waste crisis. Its suitability depends entirely on your objective.
- If your primary focus is waste management innovation: Treat pyrolysis as a key technology for processing non-mechanically-recyclable plastics, but recognize it requires robust pre-sorting and a plan for all outputs.
- If your primary focus is chemical production: View pyrolysis oil as a challenging, alternative feedstock that requires significant capital investment in purification before it can be integrated into existing infrastructure.
- If your primary focus is environmental impact assessment: Scrutinize the full lifecycle analysis, including energy inputs, logistics, and byproduct management, as the net environmental benefit is not guaranteed and varies widely by facility.
Ultimately, viewing pyrolysis as one specialized tool in a larger suite of solutions—rather than a cure-all—is the most effective path forward in tackling plastic waste.
Summary Table:
| Aspect | Key Details |
|---|---|
| Process | Thermal decomposition of plastic without oxygen (400-600°C). |
| Main Input | Low-value, mixed, or contaminated plastic waste. |
| Primary Output | Pyrolysis oil (can be refined into new plastics or fuel). |
| Key Benefit | Handles plastics that mechanical recycling cannot. |
| Main Challenge | High energy use and need for extensive oil purification. |
Ready to explore advanced recycling solutions for your laboratory or facility? KINTEK specializes in providing the equipment and expertise needed for innovative processes like pyrolysis. Whether you're researching plastic waste conversion or scaling up your operations, our lab equipment and consumables are designed to meet your precise needs. Contact our experts today to discuss how we can support your projects and help you contribute to a circular economy.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Laboratory Vortex Mixer Orbital Shaker Multifunctional Rotation Oscillation Mixer
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















