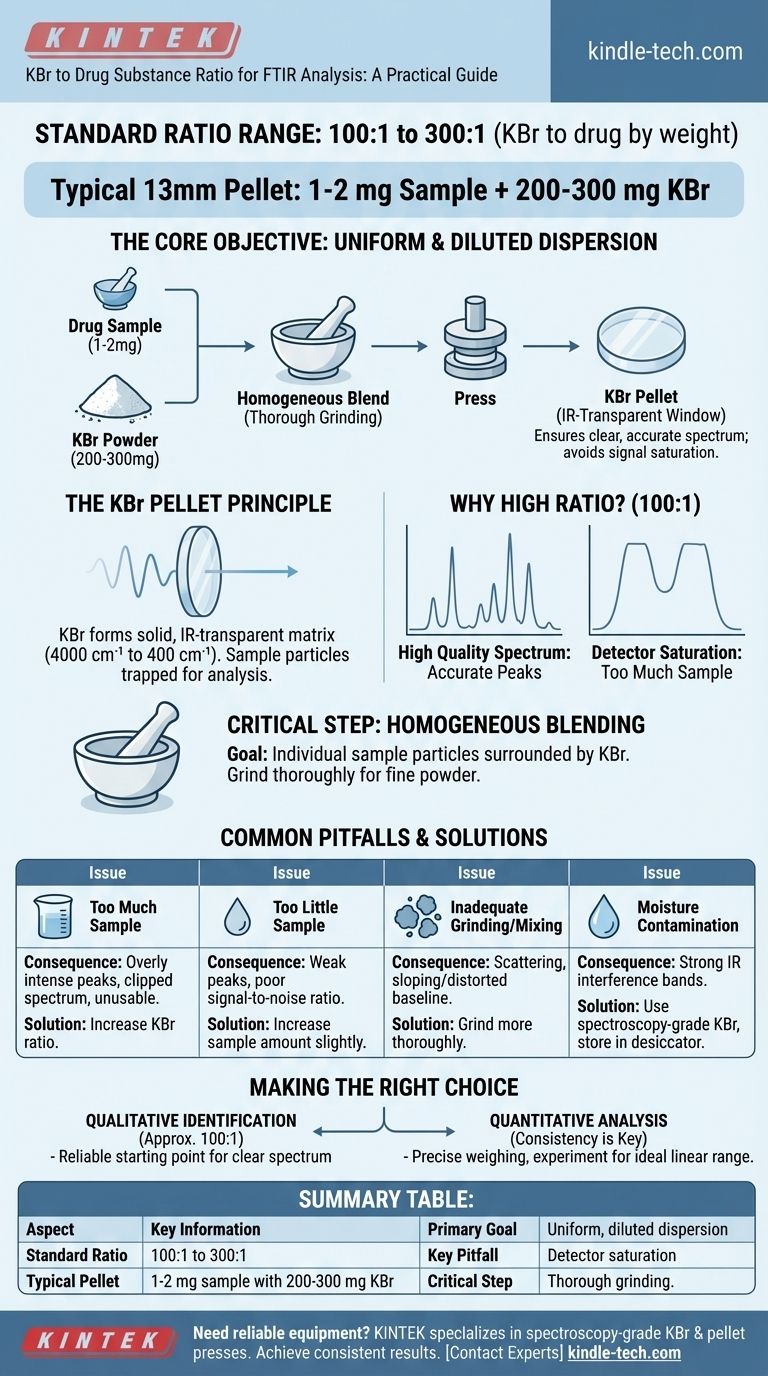
The standard ratio of potassium bromide (KBr) to a drug substance for FTIR analysis is typically between 100:1 and 300:1 by weight. For a standard 13mm pellet, this often translates to using 1-2 mg of the sample with 200-300 mg of KBr.
The core objective is not simply to achieve a specific ratio, but to create a uniform and sufficiently diluted dispersion of the sample within the KBr matrix. This ensures the resulting infrared spectrum is clear, accurate, and free from common artifacts like signal saturation.

The Principle Behind the KBr Pellet Method
The KBr pellet technique is a common sample preparation method for analyzing solid samples with FTIR spectroscopy. The entire process is designed to suspend the solid analyte in a matrix that is transparent to infrared radiation.
The Role of KBr as a Matrix
Potassium bromide (KBr) is an alkali halide salt that is transparent to infrared light over the typical analysis range (4000 cm⁻¹ to 400 cm⁻¹).
When pressed under high pressure, KBr powder forms a solid, glass-like disc. By mixing a small amount of your drug substance into the KBr, you effectively trap the sample particles within this IR-transparent window for analysis.
Why a High Ratio is Necessary
A high ratio of KBr to the sample, such as 100:1, is critical for obtaining a high-quality spectrum.
If the sample is too concentrated, the absorption of infrared light will be too strong. This causes the peaks in the spectrum to become flat-topped and broad, a phenomenon known as detector saturation. A saturated spectrum is not quantitatively accurate and can obscure important spectral features.
Achieving Uniform Dispersion
The most critical step in sample preparation is ensuring the drug substance is homogeneously blended with the KBr.
The goal is to have individual particles of your sample completely surrounded by the KBr matrix. This is typically achieved by grinding the two components together thoroughly with a mortar and pestle, ensuring a fine, consistent powder before pressing.
Understanding the Trade-offs and Common Pitfalls
While 100:1 is a standard guideline, the optimal ratio can depend on the absorptivity of your specific sample. Understanding the potential issues is key to troubleshooting your results.
Too Much Sample (Low KBr Ratio)
Using too much sample leads to overly intense absorbance peaks. The detector cannot measure the light passing through, causing peaks to appear "clipped" at the top. This makes the spectrum unusable for any form of quantitative measurement.
Too Little Sample (High KBr Ratio)
If the sample is too dilute, the resulting peaks will be very weak. This leads to a poor signal-to-noise ratio, where the characteristic peaks of your drug are difficult to distinguish from the baseline noise of the instrument.
Inadequate Grinding or Mixing
Poor mixing is a common source of error. If the sample is not finely ground and dispersed, clumps of the drug substance can cause scattering of the IR beam. This often results in a sloping or distorted baseline, making peak identification difficult.
Moisture Contamination
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has strong IR absorption bands that can interfere with your spectrum. Always use spectroscopy-grade KBr that has been kept in a desiccator or drying oven.
Making the Right Choice for Your Analysis
Your specific analytical goal will help guide your preparation.
- If your primary focus is qualitative identification: A ratio of approximately 100:1 (e.g., 2 mg of sample in 200 mg of KBr) is a reliable starting point for producing a clear, high-quality spectrum.
- If your primary focus is quantitative analysis: Consistency is paramount. You must precisely weigh your components and may need to experiment to find a ratio that keeps your primary absorption peaks within the instrument's ideal linear range.
Ultimately, the correct preparation is the one that produces a clear and reproducible spectrum with sharp, well-defined peaks for your specific substance.
Summary Table:
| Aspect | Key Information |
|---|---|
| Standard Ratio Range | 100:1 to 300:1 (KBr to drug by weight) |
| Typical Pellet (13mm) | 1-2 mg sample with 200-300 mg KBr |
| Primary Goal | Create a uniform, diluted dispersion for a clear spectrum |
| Key Pitfall to Avoid | Detector saturation from too much sample (low ratio) |
| Critical Step | Thorough grinding for homogeneous blending |
Need reliable equipment for precise FTIR sample preparation? KINTEK specializes in high-quality lab equipment and consumables, including spectroscopy-grade KBr and pellet presses. Our products help laboratories like yours achieve consistent, accurate results by ensuring perfect sample dispersion every time. Contact our experts today to find the right solution for your analytical needs!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is molding technique? A Guide to High-Volume, Complex Part Manufacturing
- What is uniaxial pressing? A Fast, Cost-Effective Powder Compaction Method
- Where are hydraulic presses used? Powering Industries from Automotive to Aerospace
- Which type of resins are used in compression molding? Thermosets vs. Thermoplastics
- What is machine pressed laminate? The Standard Manufacturing Process Explained
- How do laboratory hydraulic presses improve the molding quality of wood pellet fuel? Enhance Density and Durability
- How is a Laboratory Hydraulic Press used for Solid Electrolyte Samples? Optimize Ionic Conductivity Testing
- What compression molding is mostly used? For Large, Strong Parts from Thermosets & Composites



















