At its core, a Rotating Ring-Disk Electrode (RRDE) is an advanced electrochemical tool featuring two independent, concentric working electrodes: a central disk and an outer ring. While the disk electrode performs a primary electrochemical reaction, the ring is strategically positioned to intercept and analyze the chemical species produced at the disk. This dual-electrode setup allows for the real-time detection of reaction intermediates.
The fundamental value of the RRDE is its ability to provide mechanistic insight. It moves beyond simply measuring a reaction's overall rate (like a standard RDE) to actively "seeing" the short-lived products and intermediates generated, allowing you to understand the specific steps of a reaction pathway.

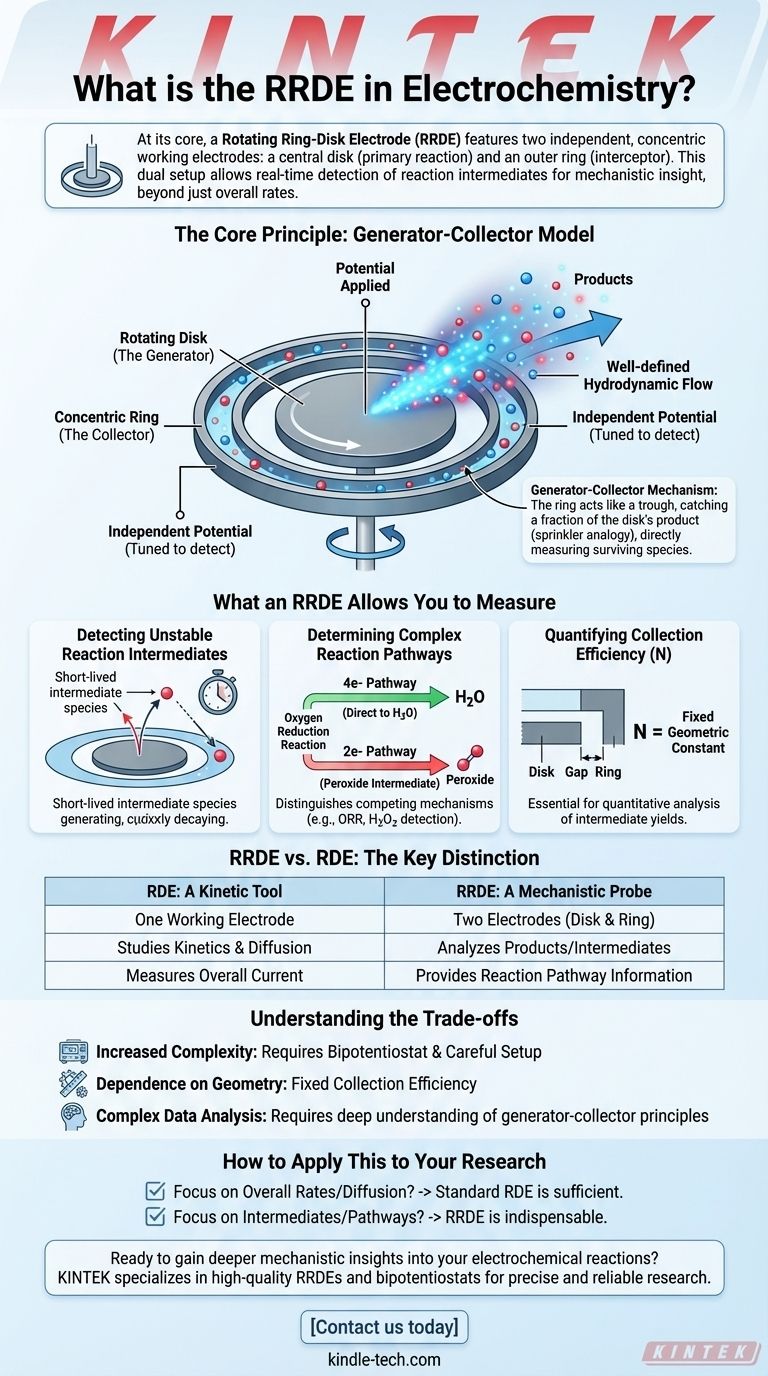
The Core Principle: How an RRDE Works
The RRDE operates based on a sophisticated "generator-collector" model, leveraging controlled fluid dynamics to probe chemical reactions as they happen.
The Rotating Disk (The Generator)
The central disk electrode functions identically to a standard Rotating Disk Electrode (RDE). It is the "generator" in the system.
A potential is applied to the disk to drive a specific electrochemical reaction. Its rotation creates a well-defined hydrodynamic flow, pulling the reactant solution towards the electrode and pushing the products radially outwards.
The Concentric Ring (The Collector)
The ring electrode is the key innovation. It is an independent electrode insulated from the disk, positioned to "collect" the products flowing away from it.
The potential of the ring can be set independently from the disk. This allows it to be tuned to specifically detect, by either oxidizing or reducing, the intermediates or final products generated at the disk.
The Generator-Collector Mechanism
Imagine the rotating disk is a spinning sprinkler head spraying out a specific chemical (the product). The ring is a circular trough placed perfectly to catch a fraction of that spray.
By measuring the current at the ring, you are directly measuring the amount of the disk's product that has survived the short journey and reached the ring. This gives you immediate information about the stability and identity of the species being generated.
What an RRDE Allows You to Measure
The dual-electrode configuration unlocks powerful analytical capabilities that are impossible with a single-electrode setup.
Detecting Unstable Reaction Intermediates
This is the primary application of RRDE. If a reaction proceeds through a short-lived intermediate, the ring can be set to a potential that specifically reacts with that intermediate, confirming its existence before it has a chance to decay or react further in the bulk solution.
Determining Complex Reaction Pathways
The RRDE is invaluable for distinguishing between competing reaction mechanisms. A classic example is the Oxygen Reduction Reaction (ORR), which is critical for fuel cells.
The ORR can proceed directly to water (a 4-electron pathway) or through a hydrogen peroxide intermediate (a 2-electron pathway). By setting the ring potential to detect peroxide, an RRDE can quantify how much of the reaction is following the less efficient 2-electron path.
Quantifying Collection Efficiency (N)
A critical parameter for any RRDE is its collection efficiency (N). This is a dimensionless number, determined by the electrode's physical geometry, that represents the fraction of material generated at the disk that is successfully captured by the ring.
Knowing this constant is essential for quantitatively analyzing the amount of intermediate produced in a reaction.
RRDE vs. RDE: The Key Distinction
While related, these two tools answer fundamentally different questions.
RDE: A Kinetic Tool
A standard Rotating Disk Electrode (RDE) has only one working electrode. It is excellent for studying the kinetics and diffusion properties of a primary electrochemical reaction by measuring the overall current.
RRDE: A Mechanistic Probe
The RRDE adds the second electrode (the ring) specifically to analyze the products of the primary reaction. This elevates it from a kinetic tool to a mechanistic probe, providing a deeper layer of information about the reaction pathway itself.
Understanding the Trade-offs
While powerful, the RRDE introduces complexity that is important to acknowledge.
Increased Experimental Complexity
An RRDE experiment requires a bipotentiostat, a device capable of controlling the potential of two working electrodes simultaneously. The setup and execution of the experiment demand more care than a standard RDE measurement.
Dependence on Geometry
The collection efficiency is fixed by the physical construction of the electrode (the size of the disk, ring, and insulating gap). This value cannot be changed during an experiment.
More Complex Data Analysis
Interpreting RRDE data requires a firm understanding of the generator-collector principles and the mathematical relationships between the disk current, ring current, and collection efficiency.
How to Apply This to Your Research
Choosing the right tool depends entirely on the question you are trying to answer.
- If your primary focus is measuring overall reaction rates or diffusion coefficients: A standard RDE is often simpler, more robust, and entirely sufficient for the task.
- If your primary focus is identifying short-lived intermediates or distinguishing between reaction pathways: The RRDE is the indispensable tool for gaining this mechanistic insight.
- If your primary focus is measuring local pH changes caused by a reaction: The ring can be used as a specialized sensor, as its potential can be set to react with H+ or OH- ions generated at the disk.
Ultimately, the RRDE provides a window into the dynamic processes occurring at the electrode surface, transforming a simple measurement into a detailed mechanistic investigation.
Summary Table:
| RRDE Component | Function | Key Insight Provided |
|---|---|---|
| Disk Electrode | Drives the primary electrochemical reaction (Generator) | Measures overall reaction kinetics |
| Ring Electrode | Detects intermediates/products from the disk (Collector) | Identifies short-lived species and reaction pathways |
| Collection Efficiency (N) | Fixed geometric constant | Enables quantitative analysis of intermediate yields |
Ready to gain deeper mechanistic insights into your electrochemical reactions?
KINTEK specializes in providing high-quality lab equipment, including RRDEs and bipotentiostats, to help researchers like you accurately detect reaction intermediates and analyze complex pathways. Whether you're developing fuel cells, studying catalysis, or probing reaction mechanisms, our tools are designed for precision and reliability.
Contact us today to discuss how our electrochemical solutions can advance your research!
Visual Guide
Related Products
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis
- What is the difference between RDE and RRDE? Unlock Advanced Electrochemical Reaction Analysis
- Why is a high-precision Rotating Ring-Disk Electrode (RRDE) essential for ORR? Unlock Precise Catalytic Kinetics
- What is the function of a Laboratory RDE system for OER catalysts? Optimize Kinetic Activity Screening
- Why Use a Three-Electrode RDE System for PEM Catalyst Screening? Master Intrinsic Kinetic Activity Analysis



















