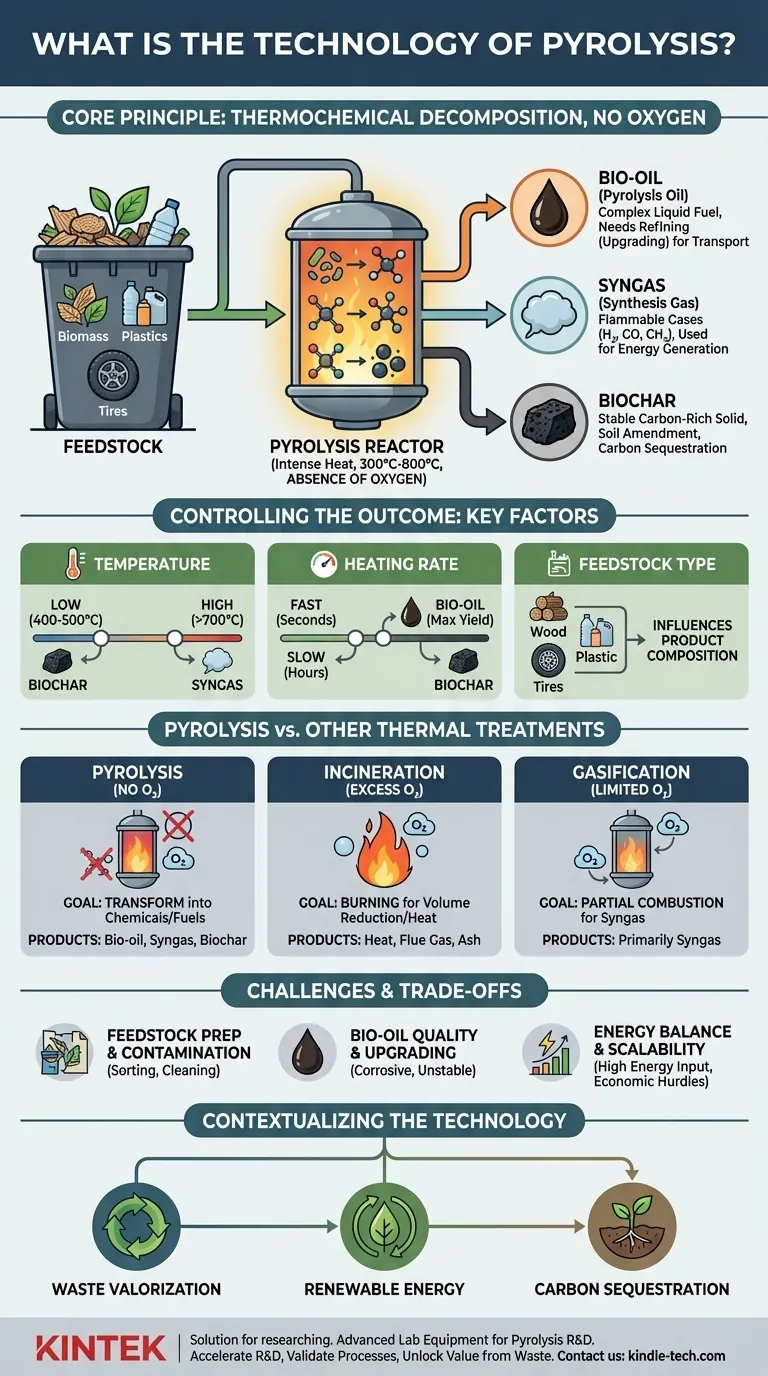
At its core, pyrolysis is the process of superheating materials in an environment completely devoid of oxygen. It is not burning, but a thermochemical decomposition that breaks down complex substances like biomass, plastics, or tires into simpler, often more valuable, components. This transformation allows us to convert what is often considered waste into useful products like liquid fuel, combustible gas, and a carbon-rich solid.
Pyrolysis should be understood not as a disposal method, but as a chemical conversion technology. By applying heat without oxygen, it deconstructs a material's chemical structure, transforming it into a distinct set of solid, liquid, and gas outputs.

How Pyrolysis Works: A Look Inside the Process
To grasp the technology, it's essential to understand its core principle, the resulting products, and the parameters that control the outcome.
The Core Principle: Heat Without Oxygen
The defining characteristic of pyrolysis is the absence of oxygen. When you heat a material with oxygen present, it combusts, or burns, releasing its energy primarily as heat and light and leaving behind ash.
By removing oxygen and creating an inert atmosphere, pyrolysis prevents combustion. Instead, the intense heat (typically ranging from 300°C to over 800°C) breaks the chemical bonds within the feedstock, creating a new mix of smaller molecules.
The Three Primary Products
The decomposition of the feedstock consistently yields three distinct product streams, the proportions of which can be manipulated.
- Bio-oil (Pyrolysis Oil): A dark, viscous liquid that is a complex blend of oxygenated organic compounds. While it has a high energy content, it is often acidic and unstable, typically requiring further refining (upgrading) before it can be used as a transport fuel.
- Syngas (Synthesis Gas): A non-condensable mixture of flammable gases, primarily hydrogen (H₂), carbon monoxide (CO), methane (CH₄), and carbon dioxide (CO₂). This gas can be combusted on-site to provide energy for the pyrolysis process itself or cleaned and used in engines or turbines.
- Biochar: A stable, carbon-rich solid that is essentially a form of charcoal. Biochar is a valuable product with applications in agriculture as a soil amendment, in filtration systems, and as a method for long-term carbon sequestration.
Key Factors Controlling the Outcome
Engineers can steer the process to favor one product over another by controlling several key variables.
- Temperature: Lower temperatures (around 400-500°C) and slower heating tend to maximize the yield of biochar. Higher temperatures (above 700°C) favor the production of syngas.
- Heating Rate: The speed at which the feedstock is heated is critical. Fast pyrolysis, involving very rapid heating for a short duration (a few seconds), is optimized to produce the highest yield of bio-oil (up to 75% by weight). Slow pyrolysis, which heats material over hours, maximizes the biochar yield.
- Feedstock Type: The chemical makeup of the input material—whether it's wood, agricultural waste, plastic, or old tires—directly influences the composition and quality of the final products.
Pyrolysis vs. Other Thermal Treatments
Understanding what pyrolysis is not is just as important as understanding what it is.
Pyrolysis vs. Incineration
Incineration is burning. It uses an excess of oxygen to fully combust waste, with the primary goal of volume reduction and heat recovery. The main outputs are heat, flue gas, and ash.
Pyrolysis, by contrast, uses no oxygen. Its goal is not to destroy the material but to transform it into new chemical products (oil, gas, char).
Pyrolysis vs. Gasification
This is a more subtle but crucial distinction. Gasification uses a limited, controlled amount of oxygen or steam. The goal is to partially combust the feedstock to maximize the production of syngas.
Pyrolysis uses zero oxygen and is therefore capable of producing a liquid fuel (bio-oil) in significant quantities, which gasification generally cannot.
Understanding the Trade-offs and Challenges
While promising, pyrolysis is not a silver bullet. A clear-eyed view of its challenges is necessary for proper application.
Feedstock Preparation and Contamination
Real-world waste streams are rarely pure. Contaminants like metals, chlorine (from PVC plastics), and excessive moisture can disrupt the process, corrode equipment, and compromise the quality of the end products. This often necessitates costly and energy-intensive pre-treatment and sorting.
Bio-oil Quality and Upgrading
Pyrolysis oil is not a "drop-in" replacement for petroleum crude oil. It is typically corrosive, chemically unstable, and contains a high amount of oxygen, which lowers its energy density. Making it suitable for use in conventional engines requires significant and expensive refining, known as upgrading.
Energy Balance and Scalability
A pyrolysis plant requires a significant energy input to reach and maintain its high operating temperatures. The process is only viable if the energy value of the products is greater than the energy consumed. Scaling a reactor from a laboratory model to an industrial facility that can process tons of material per day presents major engineering and economic hurdles.
How to Contextualize Pyrolysis Technology
To apply this knowledge, consider how pyrolysis aligns with your specific objective.
- If your primary focus is waste valorization: View pyrolysis as a powerful tool in a circular economy, capable of converting low-value waste streams (like plastics or non-recyclable biomass) into higher-value chemical products.
- If your primary focus is renewable energy: See pyrolysis as a pathway to create liquid and gaseous fuels from biomass, but be mindful that the energy balance and the need for product upgrading are critical factors for economic viability.
- If your primary focus is carbon sequestration and soil health: Focus specifically on slow pyrolysis, which is designed to maximize the production of biochar—a stable form of carbon that can improve agricultural soils and lock carbon away for centuries.
Pyrolysis is a sophisticated technology that offers a way to chemically recycle materials, but its successful implementation depends on careful engineering, a clear understanding of the feedstock, and a viable market for its unique products.
Summary Table:
| Key Aspect | Description |
|---|---|
| Core Principle | Thermochemical decomposition in the absence of oxygen. |
| Primary Products | Bio-oil (liquid fuel), Syngas (combustible gas), Biochar (solid carbon). |
| Key Variables | Temperature, heating rate (fast vs. slow pyrolysis), and feedstock type. |
| Main Advantage | Transforms waste into valuable products, enabling a circular economy. |
| Primary Challenge | Bio-oil requires upgrading; feedstock contamination and energy balance are concerns. |
Ready to explore pyrolysis solutions for your laboratory?
KINTEK specializes in advanced lab equipment for pyrolysis research and development. Whether you are developing new biofuels, studying waste valorization, or optimizing biochar production, our precise and reliable systems are designed to meet your specific needs.
We help you:
- Accelerate R&D with equipment that enables precise control over temperature and heating rates.
- Validate Processes with reliable data to scale your technology from lab to pilot plant.
- Unlock Value from waste streams by providing the tools needed for efficient chemical conversion.
Contact us today to discuss how our pyrolysis laboratory equipment can support your innovation goals. Let's turn your research into reality.
Visual Guide
Related Products
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Laboratory Test Sieves and Sieving Machines
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the temperature range of a graphite furnace? Unlock up to 3000°C for advanced materials processing.
- What are the disadvantages of graphite furnace? Key Limitations and Operational Costs
- What is the graphite furnace method? Achieve Ultra-High Temperatures with Purity & Speed
- What is the use of graphite furnace? Achieve Extreme-Temperature Processing for Advanced Materials
- Why is a high-vacuum graphite heating element furnace used for HAp sintering? Achieve Pure, High-Bond Coatings



















