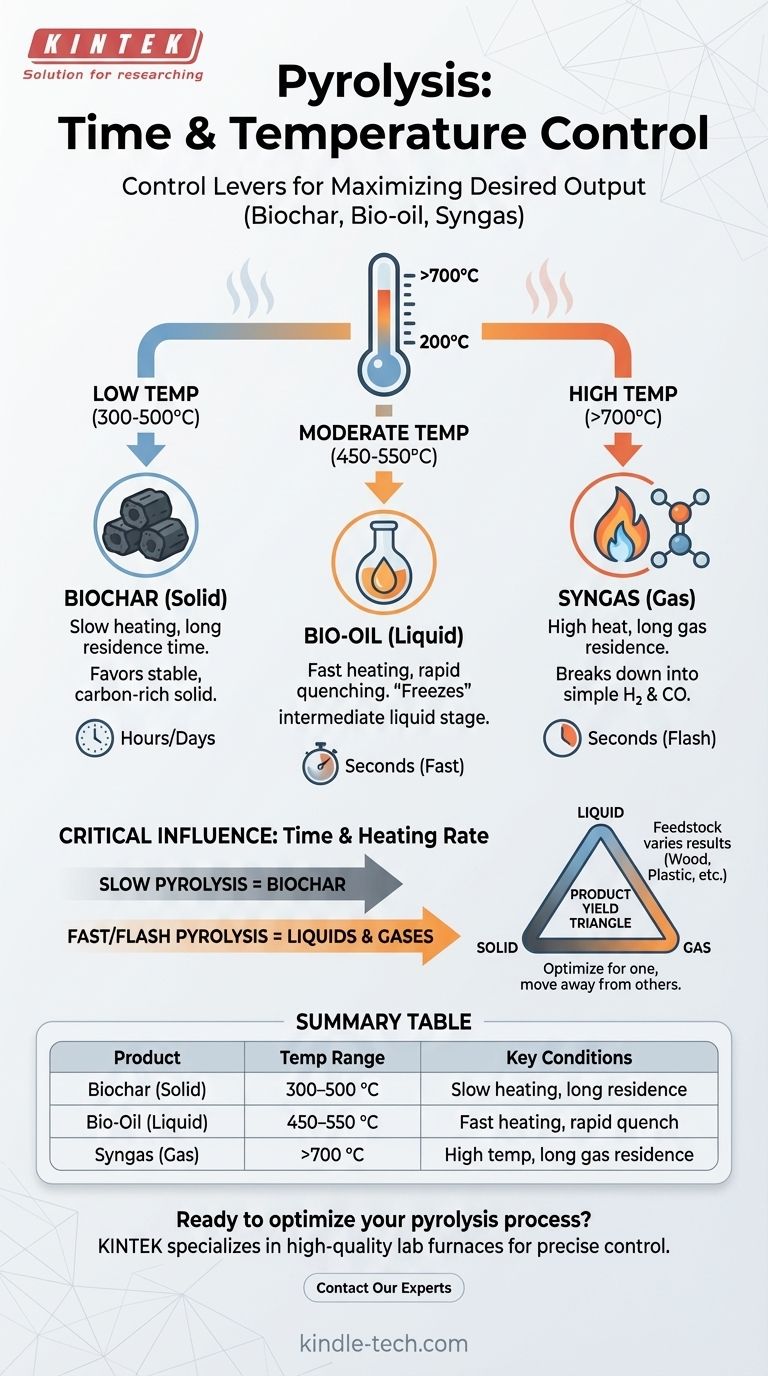
In short, pyrolysis does not have one single time and temperature. Instead, it occurs across a wide range of conditions, typically starting between 200–300 °C (390–570 °F) for organic materials like wood. The specific time and temperature you use are the most critical factors that determine the final products of the process.
The core principle to understand is that pyrolysis temperature and time are not fixed values, but rather control levers. You adjust these parameters specifically to maximize your desired output, whether it's a solid (biochar), a liquid (bio-oil), or a gas (syngas).

The Role of Temperature in Pyrolysis
Temperature is the primary driver that dictates the chemical breakdown of the feedstock. By controlling the heat, you control the end product.
Low Temperatures for Solid Products (Biochar)
At lower temperatures, generally in the range of 300–500 °C, the pyrolysis process is slower. This condition favors the production of char, a stable, carbon-rich solid also known as biochar or charcoal.
The slower decomposition allows carbon atoms to arrange into stable aromatic structures, leaving a solid residue behind instead of breaking down further into liquids and gases.
Moderate Temperatures for Liquid Products (Bio-oil)
To maximize the yield of liquid products, known as bio-oil or pyrolysis oil, a moderate temperature range of roughly 450-550 °C is typically used.
Crucially, this process requires not only the right temperature but also a very rapid heating rate and immediate cooling (quenching) of the resulting vapors. This rapid change "freezes" the chemical reactions at the intermediate liquid stage before they can break down further into gas.
High Temperatures for Gas Products (Syngas)
At high temperatures, often above 700 °C, the process favors the production of non-condensable gases. This mixture is known as syngas (synthesis gas), primarily composed of hydrogen (H₂) and carbon monoxide (CO).
The intense heat provides enough energy to break down virtually all the complex organic molecules, including any intermediate liquids, into the simplest possible gaseous molecules.
The Critical Influence of Time and Heating Rate
The time the material is held at temperature (residence time) and the speed at which it is heated are just as important as the temperature itself.
Slow Pyrolysis for Biochar
This process involves very slow heating rates and long residence times (hours or even days). This gives the feedstock ample time to slowly convert into charcoal, maximizing the solid product yield.
Fast and Flash Pyrolysis for Liquids and Gases
Fast pyrolysis involves heating the material extremely quickly (in seconds) to the target temperature. This is essential for maximizing bio-oil, as it rapidly vaporizes the material which is then quickly condensed.
If the goal is syngas, a fast heating rate followed by a longer gas residence time at high temperature ensures the complete thermal cracking of all components into gas.
Understanding the Trade-offs
You cannot optimize for all outputs simultaneously. Understanding the inherent trade-offs is key to controlling the process effectively.
The Product Yield Triangle
Think of the three products—solid, liquid, and gas—as points on a triangle. Pushing the process conditions to favor one corner (e.g., high-yield biochar) necessarily moves you away from the others (lower yields of oil and gas).
Your goal determines the parameters. There is no universally "best" setting, only the best setting for a specific desired outcome.
Feedstock Is a Key Variable
The exact temperatures and yields will also depend heavily on the feedstock being used. Wood, plastic, agricultural waste, and tires all have different chemical compositions and will therefore behave differently under pyrolysis. The parameters must be tuned for the specific material you are processing.
Choosing the Right Parameters for Your Goal
To apply this knowledge, first define your desired product. Then, select the process conditions that favor its creation.
- If your primary focus is producing high-quality biochar: Use slow pyrolysis with a low heating rate and a final temperature between 300-500 °C.
- If your primary focus is maximizing bio-oil yield: Use fast pyrolysis with a very high heating rate to a moderate temperature (around 500 °C) and ensure you can rapidly quench the vapors.
- If your primary focus is generating syngas: Use fast or flash pyrolysis at high temperatures, typically above 700 °C, to ensure complete breakdown of all organic matter.
Ultimately, mastering pyrolysis is about understanding how to manipulate time and temperature to navigate these trade-offs and reliably produce your intended result.
Summary Table:
| Desired Product | Typical Temperature Range | Key Process Conditions |
|---|---|---|
| Biochar (Solid) | 300–500 °C | Slow heating rate, long residence time |
| Bio-Oil (Liquid) | 450–550 °C | Fast heating, rapid vapor quenching |
| Syngas (Gas) | >700 °C | High temperature, long gas residence time |
Ready to optimize your pyrolysis process? The right lab equipment is essential for precise temperature and time control. KINTEK specializes in high-quality lab furnaces and pyrolysis systems designed to help you reliably produce your target product—whether it's biochar, bio-oil, or syngas. Contact our experts today to find the perfect solution for your laboratory's needs!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Mesh belt controlled atmosphere furnace
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas



















