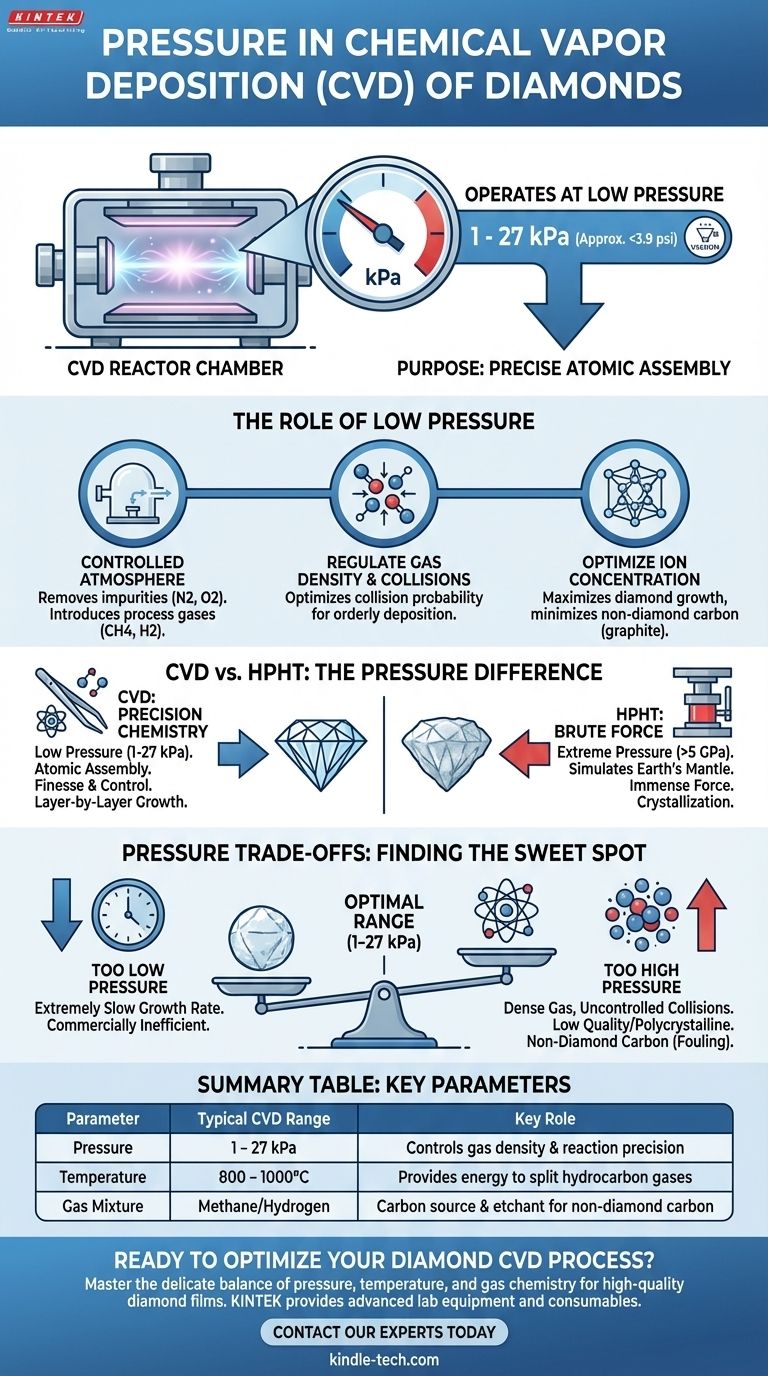
In stark contrast to other methods, the chemical vapor deposition (CVD) of diamonds operates under relatively low pressure. The process typically requires a pressure well below that of our normal atmosphere, generally in the range of a few kilopascals (kPa) up to about 27 kPa (approximately 3.9 psi).
The critical insight is not the specific pressure value, but the purpose behind it. Diamond CVD relies on creating a near-vacuum environment to precisely control chemical reactions, assembling a diamond atom-by-atom, rather than using immense force to crush carbon into a diamond structure.

The Role of Low Pressure in CVD
The pressure inside a CVD reactor is one of the most critical variables. It's not just about creating a vacuum; it's about engineering the perfect environment for high-quality diamond to form on a substrate.
Creating a Controlled Atmosphere
The process begins by evacuating the deposition chamber to remove atmospheric gases like nitrogen and oxygen, which would interfere with the reaction. This creates a clean, controlled environment into which specific process gases (typically methane and hydrogen) are introduced.
Regulating Gas Density and Collisions
The chosen low pressure directly determines the density of the gas molecules. This is crucial for controlling the probability of collisions between them. The pressure must be just right to allow carbon-containing gas molecules to break apart and deposit onto the diamond seed crystal in an orderly fashion.
Optimizing Ion Concentration
The goal is to maximize the concentration of specific atomic groups required for diamond growth while minimizing the formation of non-diamond carbon, such as graphite. The pressure range of several to dozens of kPa is a "sweet spot" that enables high-quality diamond film deposition with an efficient growth rate.
Why Low Pressure Defines the CVD Method
The use of low pressure is the fundamental difference between the two primary methods of creating lab-grown diamonds: CVD and High-Pressure/High-Temperature (HPHT). Understanding this distinction is key to understanding the processes themselves.
CVD: Precision Chemistry
CVD is a process of "atomic assembly." In the low-pressure chamber, energy (often from microwaves) is used to split hydrocarbon gas molecules. These carbon atoms then deposit onto a substrate, or "seed crystal," slowly building up the diamond's lattice structure layer by layer. It is a process of finesse and chemical control.
HPHT: Simulating Earth's Mantle
The HPHT method, by contrast, uses brute force. It mimics the natural conditions deep within the Earth where diamonds form. A carbon source material is subjected to immense pressures (over 5 GPa) and extreme temperatures (around 1500°C), forcing the carbon atoms to crystallize into a diamond.
Understanding the Pressure Trade-offs
The pressure in a CVD system is a delicate balancing act. Deviating from the optimal range can significantly compromise the final product.
If Pressure is Too Low
If the pressure is significantly below the optimal range, the density of the reactant gas becomes too low. This results in an extremely slow growth rate, making the process commercially inefficient.
If Pressure is Too High
If the pressure is too high, the gas becomes too dense. This increases the frequency of uncontrolled collisions, which can lead to the formation of lower-quality polycrystalline diamonds or, worse, non-diamond carbon forms like graphite. This "fouling" of the crystal compromises its clarity and structural integrity.
Interplay with Other Variables
Pressure does not work in isolation. The ideal pressure setting is tightly coupled with the temperature (typically 800-1000°C) and the precise ratio of methane to hydrogen gas in the chamber. A successful diamond growth requires fine-tuning all these variables in concert.
Making the Right Choice for Your Goal
Your understanding of pressure in diamond CVD depends on your ultimate objective.
- If your primary focus is understanding the basic principle: Remember that CVD uses low pressure to enable precise chemical assembly, which is the direct opposite of the brute-force, high-pressure HPHT method.
- If your primary focus is process optimization: The ideal pressure is a critical "sweet spot" (typically 1-27 kPa) that must be carefully balanced with temperature and gas mixture to maximize both growth rate and crystal quality.
Ultimately, mastering pressure is about mastering the control needed to build one of the world's hardest materials one atom at a time.
Summary Table:
| Parameter | Typical CVD Range | Key Role |
|---|---|---|
| Pressure | 1 - 27 kPa | Controls gas density & reaction precision for high-quality diamond growth |
| Temperature | 800 - 1000°C | Provides energy to split hydrocarbon gases |
| Gas Mixture | Methane/Hydrogen | Provides carbon source and etchant for non-diamond carbon |
Ready to Optimize Your Diamond CVD Process?
Understanding the precise interplay of pressure, temperature, and gas chemistry is the key to achieving high-quality, consistent diamond films. KINTEK specializes in providing the advanced lab equipment and consumables necessary to master this delicate balance.
Whether you are setting up a new research line or optimizing an existing process, our expertise in CVD technology can help you:
- Achieve superior crystal quality and growth rates
- Reduce process variability and material waste
- Scale your diamond synthesis from research to production
Don't let process variables limit your innovation. Contact our experts today to discuss how KINTEK's solutions can accelerate your diamond research and development.
Visual Guide
Related Products
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- Are CVD diamonds synthetic? Discover the Truth About Lab-Grown Diamonds
- What is MPCVD? Unlock Atom-by-Atom Precision for High-Purity Materials
- What is CVD diamond? The Ultimate Guide to Lab-Grown Diamonds and Their Uses
- What is CVD diamond technology? Grow High-Quality, Engineered Diamonds for Your Applications
- What is better lab grown or natural diamonds? A Guide to Choosing Your Perfect Stone
- What do you need to grow lab diamonds? Carbon, Seed, and Immense Energy Explained
- What are the applications of microwave plasma? From Diamond Synthesis to Semiconductor Fabrication
- What is the future value of lab grown diamond? Understanding Its Depreciating Financial Worth



















