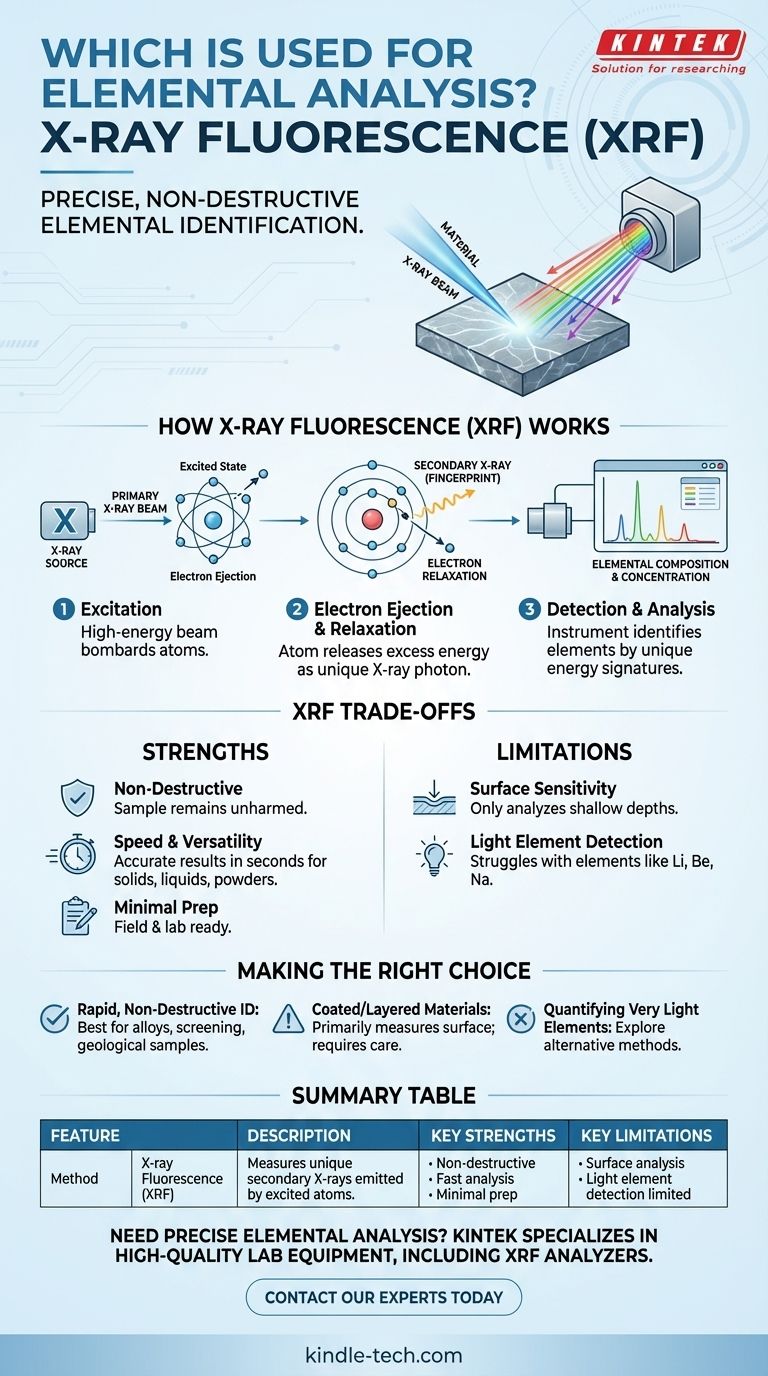
For precise elemental analysis, one of the most widely used and trusted methods is X-ray Fluorescence, commonly known as XRF. This non-destructive technique identifies the chemical elements within a sample by bombarding it with X-rays. Each element then responds by emitting its own unique, characteristic X-ray "fingerprint," allowing for accurate identification and quantification without damaging the material being tested.
X-ray Fluorescence (XRF) provides a powerful solution for determining the elemental composition of a material. It operates by exciting atoms and measuring their unique energy signatures, making it an indispensable tool for everything from quality control to geological exploration.

How X-ray Fluorescence (XRF) Works
To understand the value of XRF, you must first grasp its underlying principle. The process is a rapid, three-step chain of events at the atomic level.
The Principle of Excitation
First, a primary X-ray beam, generated by an instrument, is directed at the surface of the sample. This high-energy beam bombards the atoms within the material, transferring a significant amount of energy to them.
Electron Ejection and Relaxation
This incoming energy is potent enough to knock an electron out of one of the atom's inner orbital shells, creating a vacancy. This leaves the atom in an unstable, excited state. To regain stability, an electron from a higher-energy outer shell immediately drops down to fill the void.
The Characteristic "Fingerprint"
As the outer electron falls into the lower energy position, the atom must release the excess energy. It does this by emitting a secondary X-ray photon. The energy of this emitted photon is precisely equal to the difference in energy between the two orbital shells, a value that is unique and characteristic for every single element.
Detection and Analysis
A detector within the XRF analyzer measures the energy and number of these emitted secondary X-rays. By identifying the specific energy levels, the instrument confirms which elements are present. By counting the number of X-rays at each energy level, it can also determine the concentration of each element in the sample.
Understanding the Trade-offs of XRF
Like any analytical technique, XRF has distinct advantages and limitations that make it suitable for some applications but not others.
Strength: Non-Destructive Analysis
The most significant advantage of XRF is that it is non-destructive. The sample is not harmed, altered, or consumed during the analysis. This is critical when testing valuable or unique items like archeological artifacts, jewelry, or critical machinery components.
Strength: Speed and Versatility
XRF analyzers, particularly handheld models, can deliver accurate results in seconds. They can be used in the field, on a production line, or in a lab to analyze solids, liquids, powders, and alloys with minimal sample preparation.
Limitation: Surface Sensitivity
XRF is primarily a surface analysis technique. The primary X-rays only penetrate a shallow depth into the material (from micrometers to millimeters, depending on the material's density). If the sample is not homogenous, the surface reading may not represent the bulk composition of the object's interior.
Limitation: Light Element Detection
Standard XRF instruments struggle to accurately detect very light elements (such as lithium, beryllium, and sodium). The characteristic X-rays these elements emit have very low energy, making it difficult for them to escape the sample and be measured by the detector.
Making the Right Choice for Your Goal
Selecting the correct method depends entirely on your objective. XRF is a powerful tool when used in the right context.
- If your primary focus is rapid, non-destructive material identification: XRF is an exceptional choice, especially for verifying metal alloys, screening for hazardous substances, or analyzing geological samples.
- If your primary focus is analyzing a coated or layered material: Be aware that XRF will primarily measure the surface layer, and you may need other techniques to understand the underlying composition.
- If your primary focus is quantifying very light elements: You should investigate alternative methods, as standard XRF is not optimized for this specific task.
By understanding the core principles and trade-offs of XRF, you can confidently determine when it is the right tool to solve your analytical challenge.
Summary Table:
| Feature | Description |
|---|---|
| Method | X-ray Fluorescence (XRF) |
| Principle | Measures unique secondary X-rays emitted by excited atoms. |
| Key Strength | Non-destructive; does not damage the sample. |
| Key Strength | Fast analysis (seconds) for solids, liquids, and powders. |
| Key Limitation | Primarily a surface analysis technique. |
| Key Limitation | Limited detection of very light elements (e.g., Lithium, Beryllium). |
Need precise, non-destructive elemental analysis for your laboratory?
KINTEK specializes in providing high-quality lab equipment, including XRF analyzers, to meet your exact needs. Whether you're in quality control, geology, or materials science, our solutions deliver the speed, accuracy, and reliability your work demands.
Contact our experts today to find the perfect analytical tool for your application!
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Customizable XRD Sample Holders for Diverse Research Applications
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- What is the temperature of brazing alloys? Mastering the Critical Liquidus Point for Strong Joints
- What is the physical significance of the innermost boundary layer? Crucial Insights for Material Containment
- What is the theory and practice of RF sputtering? Master Thin-Film Deposition for Insulating Materials
- Is deposition physical or chemical? Unraveling the Science of Phase Transitions
- Does metal expand on heating or cooling? The Science of Thermal Expansion Explained
- What does the acronym CVD stand for? Decoding Its Meaning in Medicine and Technology
- Is deposition technology an amazing scientific advancement? The Unsung Art of Building Our World
- What are 3 benefits of biomass energy? Turn Waste into Renewable Power
















