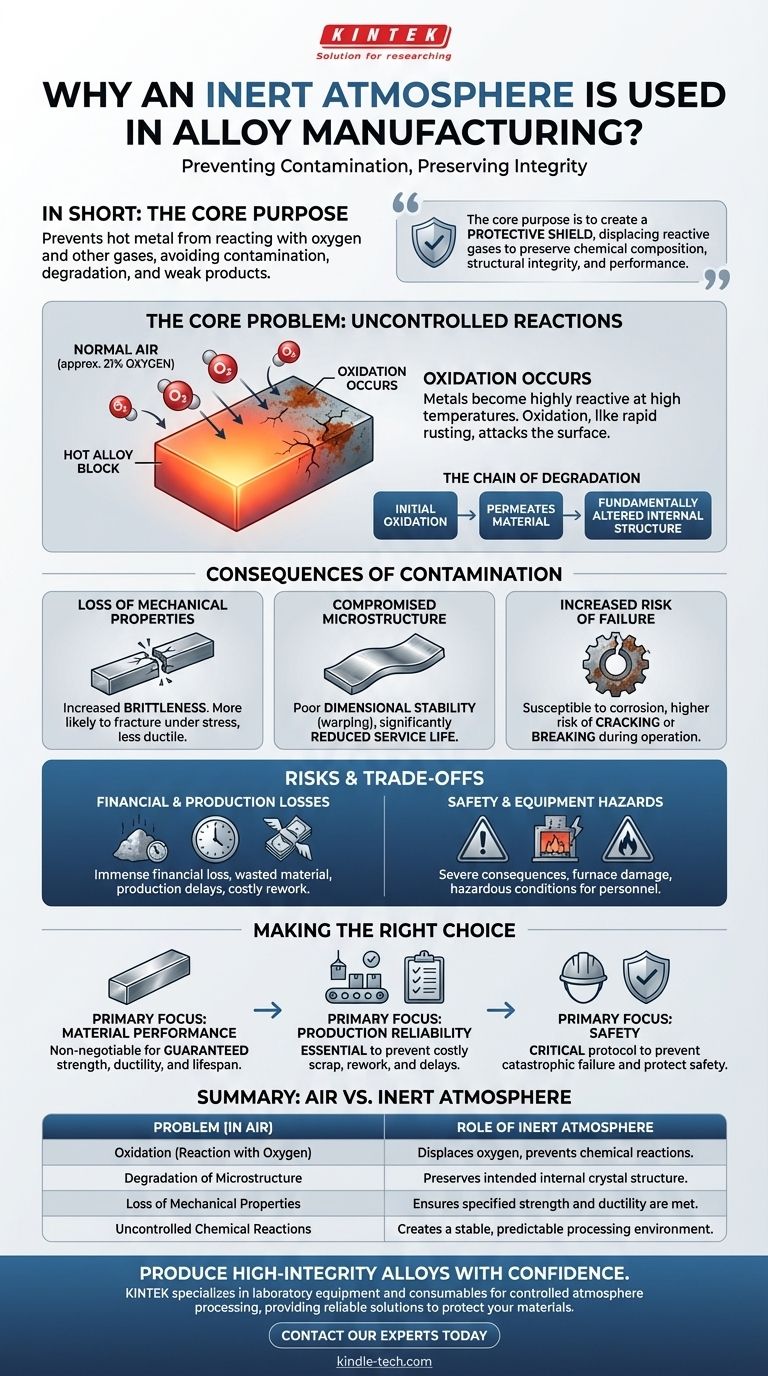
In short, an inert atmosphere is used during alloy manufacturing to prevent the hot metal from reacting with oxygen and other gases in the air. These reactions, primarily oxidation, contaminate the alloy, severely degrading its fundamental properties and leading to a weak, unreliable final product.
The core purpose of using an inert atmosphere is to create a protective shield around the alloy. This shield displaces reactive gases like oxygen, preserving the material's intended chemical composition, structural integrity, and performance characteristics during heat treatment.

The Core Problem: Uncontrolled Atmospheric Reactions
During manufacturing processes like heat treatment, alloys are heated to very high temperatures. At these temperatures, the metals become highly reactive and vulnerable to their surrounding environment.
What Happens in Normal Air?
Normal air is composed of roughly 21% oxygen. When a hot alloy is exposed to this oxygen, a chemical reaction called oxidation occurs on its surface.
This is the same fundamental process that causes iron to rust, but it happens much more rapidly and aggressively at the high temperatures used in manufacturing.
The Chain of Degradation
This initial oxidation is not just a surface-level issue. It triggers a cascade of negative effects that permeate the material, fundamentally altering its internal structure and behavior.
Contamination from the atmosphere compromises the alloy from the moment it is created.
The Consequences of Contamination
Failing to control the furnace atmosphere leads to a product that cannot be trusted. The damage manifests in several critical ways.
Loss of Mechanical Properties
Contamination directly attacks the alloy's strength and ductility. The material becomes more brittle, meaning it is more likely to fracture or crack under stress instead of bending or deforming.
Compromised Microstructure
The carefully designed internal crystal structure of the alloy is disrupted. This leads to poor dimensional stability, where the part may warp or change shape unexpectedly, and a significantly reduced service life.
Increased Risk of Failure
An oxidized alloy is more susceptible to long-term failure. It will corrode more easily and is at a much higher risk of cracking or breaking during operation, even under normal loads.
Understanding the Trade-offs and Risks
The decision to use an inert atmosphere is not just about quality; it's also about managing significant operational risks.
Financial and Production Losses
A single contaminated batch can result in immense financial loss. The material is wasted, production schedules are delayed, and significant resources are spent on rework or disposal.
Safety and Equipment Hazards
The consequences of an uncontrolled atmosphere can be severe. In some cases, unwanted chemical reactions can damage the furnace itself or create hazardous conditions that pose a direct risk to employee safety.
Making the Right Choice for Your Goal
Using an inert atmosphere is a foundational requirement for producing high-integrity alloys. The specific motivation may change, but the need remains constant.
- If your primary focus is material performance: An inert atmosphere is non-negotiable to guarantee the alloy meets its specified strength, ductility, and lifespan.
- If your primary focus is production reliability: Proper atmospheric control is essential to prevent the high costs associated with scrap, rework, and production delays.
- If your primary focus is safety: Controlling the furnace atmosphere is a critical safety protocol to prevent catastrophic material failure and protect both personnel and equipment.
Ultimately, an inert atmosphere ensures the alloy you create is the alloy you designed.
Summary Table:
| Problem (in Air) | Consequence for Alloy | Role of Inert Atmosphere |
|---|---|---|
| Oxidation (Reaction with Oxygen) | Surface contamination, brittleness | Displaces oxygen, prevents chemical reactions |
| Degradation of Microstructure | Warping, reduced service life, cracking | Preserves intended internal crystal structure |
| Loss of Mechanical Properties | Weak, unreliable final product | Ensures specified strength and ductility are met |
| Uncontrolled Chemical Reactions | Scrap, rework, safety hazards | Creates a stable, predictable processing environment |
Produce high-integrity alloys with confidence. Contamination from atmospheric gases like oxygen can lead to brittle, weak materials and costly production failures. KINTEK specializes in laboratory equipment and consumables for controlled atmosphere processing, providing the reliable solutions you need to protect your materials during critical heat treatment stages.
Contact our experts today to discuss how we can help you achieve superior alloy performance, reduce scrap rates, and ensure the safety of your operations.
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What defines a commercial nitrogen-based atmosphere? Gain Precision and Flexibility in Your Heat Treatment Process
- What is an inert atmosphere heat treatment? Protect Your Metals from Oxidation & Decarburization
- How does a hydrogen atmosphere control system influence the formation of pores in Cu-Ni microtubes? Expert Insights
- What is the function of an atmosphere furnace in TG-QMS analysis? Unlock Precise Battery Material Testing
- How do atmosphere heat treatment furnaces and chemical activators function together? Optimize Silicide Coatings
- What are the hazards of inert gases? Understanding the Silent Threat of Asphyxiation
- What is nitriding in heat treatment? Enhance Durability with Precision Surface Hardening
- Why must UO2 pellets undergo heat treatment in a reducing atmosphere furnace? Ensure Experimental Precision



















