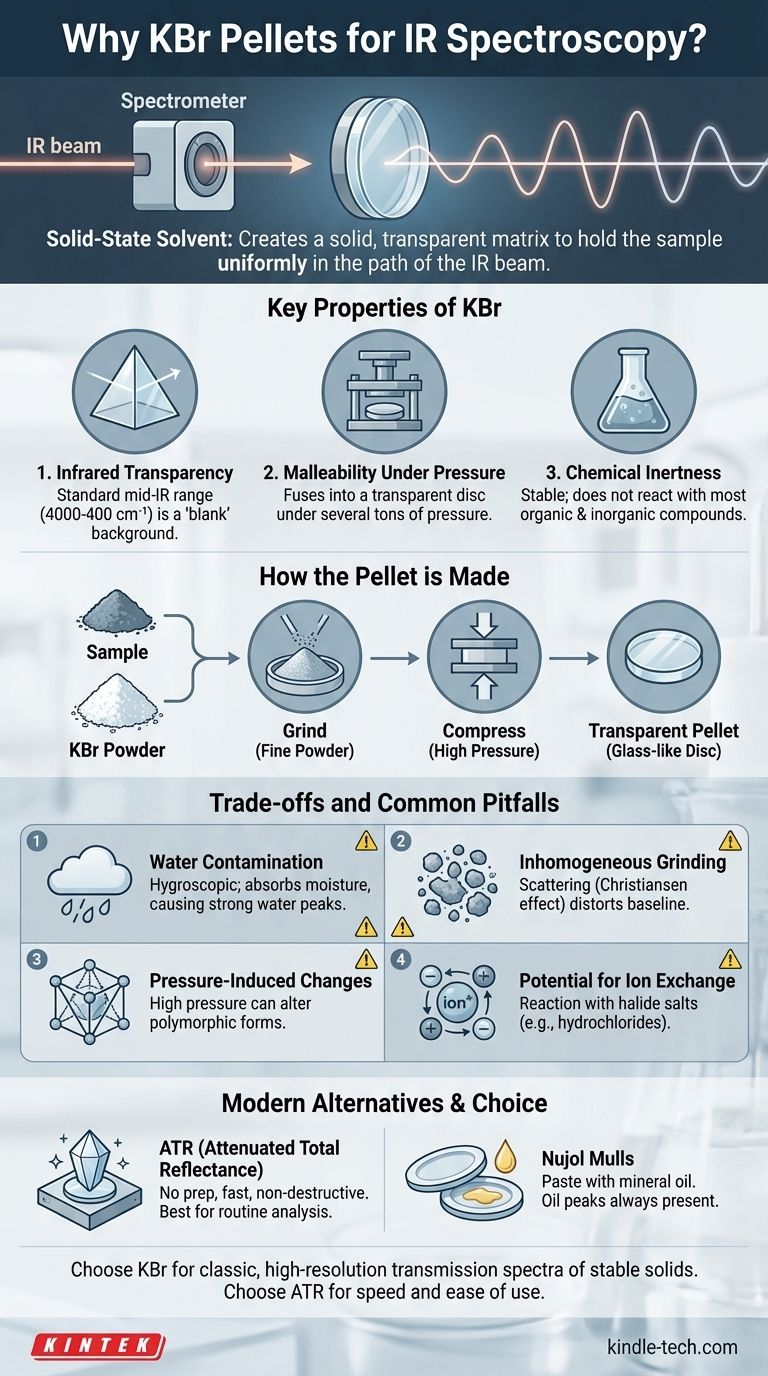
In infrared (IR) spectroscopy, Potassium Bromide (KBr) is used because it acts as an ideal solid-state solvent for the sample being analyzed. Its primary function is to create a solid, transparent matrix that holds the sample uniformly in the path of the spectrometer's IR beam. KBr is chosen specifically because it does not absorb light in the mid-infrared region, ensuring that the resulting spectrum is purely from the sample, not the KBr itself.
The core challenge in analyzing solid samples with IR spectroscopy is holding them in the beam path without the holder itself interfering with the measurement. KBr pellets solve this by creating a transparent "window" in the infrared range, allowing the spectrometer to see only the chemical bonds of the substance being studied.

The Purpose of a Sample Matrix
Infrared spectroscopy measures the vibration of chemical bonds when they absorb IR radiation. To get a clean and readable spectrum from a solid sample, the sample must be prepared correctly.
Why a Matrix is Necessary
For transmission IR spectroscopy, the infrared beam must pass through the sample. If you use a chunk of a solid organic compound, it's usually too thick and will absorb all the light, resulting in a useless, flat-lined spectrum.
The sample must be made very dilute and dispersed evenly to allow a measurable amount of light to pass through. A matrix like KBr provides the medium for this dilution.
How the Pellet is Made
The solid sample is ground into an extremely fine powder with a much larger amount of pure, dry KBr powder. This mixture is then placed into a die and compressed under immense pressure (several tons).
Under this pressure, the KBr exhibits plastic flow, causing its crystalline particles to fuse together into a solid, glass-like, transparent disc or "pellet," with the sample particles trapped inside.
Key Properties of KBr
KBr is not the only material that can be used, but its combination of properties makes it a near-perfect choice for general-purpose solid sampling.
1. Infrared Transparency
This is the most critical property. KBr is an ionic salt with no molecular vibrations that absorb light in the standard mid-IR range (4000 cm⁻¹ to 400 cm⁻¹). This means it creates a "blank" background, so every peak you see in the final spectrum can be attributed to your sample.
2. Malleability Under Pressure
The ability of KBr to deform and fuse into a transparent disc under pressure is a unique physical property that makes the pellet technique possible. Other salts might shatter or remain opaque.
3. Chemical Inertness
KBr is a stable salt that does not react with the vast majority of organic and inorganic compounds. This ensures that you are measuring the original sample, not a new compound formed by a reaction inside the pellet.
Understanding the Trade-offs and Common Pitfalls
While KBr is an excellent tool, it is not without its challenges. Proper technique is critical to avoid generating a poor-quality or misleading spectrum.
The Problem of Water Contamination
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong, broad IR absorptions around 3400 cm⁻¹ (O-H stretch) and 1640 cm⁻¹ (H-O-H bend).
If your KBr is not perfectly dry, these large water peaks can obscure important peaks from your sample, especially those from -OH or -NH groups. This is why KBr must be stored in a desiccator and often baked in an oven before use.
Inhomogeneous Sample Grinding
If the sample is not ground into particles smaller than the wavelength of the IR light, significant light scattering can occur. This phenomenon, known as the Christiansen effect, results in a distorted, sloping baseline that can make the spectrum difficult to interpret accurately.
Pressure-Induced Changes
For some sensitive crystalline materials, the high pressure used to form the pellet can induce a change in the sample's polymorphic form (its crystal structure). This can result in a spectrum that does not represent the original, un-pressed material.
Potential for Ion Exchange
When analyzing certain salts, particularly halide salts like hydrochlorides (R-NH₃⁺Cl⁻), KBr can participate in ion exchange. The bromide ion can displace the chloride ion, creating a mixture of salts within the pellet and producing a confusing spectrum that is not representative of the pure starting material.
When KBr Isn't the Right Choice: Modern Alternatives
The KBr pellet technique, while a classic method, has been largely superseded in many modern labs by a simpler technique.
Attenuated Total Reflectance (ATR)
ATR is the most common sampling method used today. It involves placing the solid or liquid sample directly onto a small, hard crystal (often diamond or zinc selenide). The IR beam is passed through the crystal in such a way that it interacts with the surface of the sample.
ATR requires virtually no sample preparation, is non-destructive, and is unaffected by water contamination in the same way as KBr. It is faster, easier, and more reliable for routine analysis.
Nujol Mulls
Before ATR became common, the primary alternative to KBr was a Nujol mull. The sample is ground with a few drops of mineral oil (Nujol) to create a thick paste. This paste is then spread between two salt plates (like NaCl or KBr). The main disadvantage is that the mineral oil itself has C-H absorption peaks that will always be present in the spectrum.
Making the Right Choice for Your Goal
Selecting the correct sample preparation method is crucial for obtaining meaningful data from your IR spectrometer.
- If your primary focus is obtaining a classic, high-resolution transmission spectrum for a stable solid: A carefully prepared KBr pellet remains a gold standard for archival-quality data.
- If your primary focus is speed, ease of use, and routine analysis: ATR spectroscopy is the undisputed modern choice for both solids and liquids.
- If your sample is sensitive to pressure or may react with KBr: Consider using a Nujol mull or, more practically, the ATR method.
- If you need to analyze a thin polymer sheet or film: It is often best to mount the film directly in the IR beam path without any matrix at all.
Ultimately, understanding these sampling principles empowers you to select the technique that best reveals the true chemical identity of your material.
Summary Table:
| Aspect | Benefit | Consideration |
|---|---|---|
| Infrared Transparency | No interference in mid-IR range (4000-400 cm⁻¹) | Ensures spectrum reflects only the sample |
| Malleability | Fuses under pressure into a transparent disc | Requires high-pressure equipment |
| Chemical Inertness | Does not react with most samples | Avoids ion exchange with certain salts |
| Common Pitfalls | — | Hygroscopic (absorbs water); requires dry handling |
Need precise IR spectroscopy solutions for your lab? KINTEK specializes in high-quality lab equipment and consumables, including reliable KBr pellet presses and accessories tailored for accurate solid sample analysis. Whether you're preparing classic KBr pellets or exploring modern ATR techniques, our products ensure clear, contamination-free spectra. Contact us today to enhance your IR sampling process and achieve reliable results!
Visual Guide
Related Products
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
People Also Ask
- What role does a laboratory hydraulic press play in sulfide-based solid-state batteries? Achieve Optimal Densification
- What was wrong with the hydraulic press? Key Drawbacks in Maintenance and Safety
- How do laboratory hydraulic presses and forming molds create 3D superlattice nanocatalysts? Enhance Material Density
- Is hydraulic fluid environmentally safe? Discover the Truth About Eco-Friendly Alternatives
- What is the XRF method widely used to measure? Get Fast, Non-Destructive Elemental Analysis
- What is the purpose of KBr pellets? Unlock Clear FTIR Analysis of Solid Samples
- What are the major parts of a press? A Guide to the Core Components of a Hydraulic Press
- Can a hydraulic fluid lose this property if it gets too hot from too much pressure? Protect Your System from Heat Damage



















