At its core, a controlled atmosphere furnace is desirable for sintering because it gives you absolute command over the chemical environment. This control is not a minor feature; it is fundamental to preventing unwanted reactions like oxidation and contamination from atmospheric gases. By eliminating these variables, you can produce high-purity, dense, and high-performance components with properties that would be impossible to achieve in open air.
Sintering in ambient air is a process of compromise. A controlled atmosphere furnace removes this compromise by eliminating reactive gases, allowing for the formation of materials with superior density, purity, and structural integrity that would be impossible to achieve otherwise.

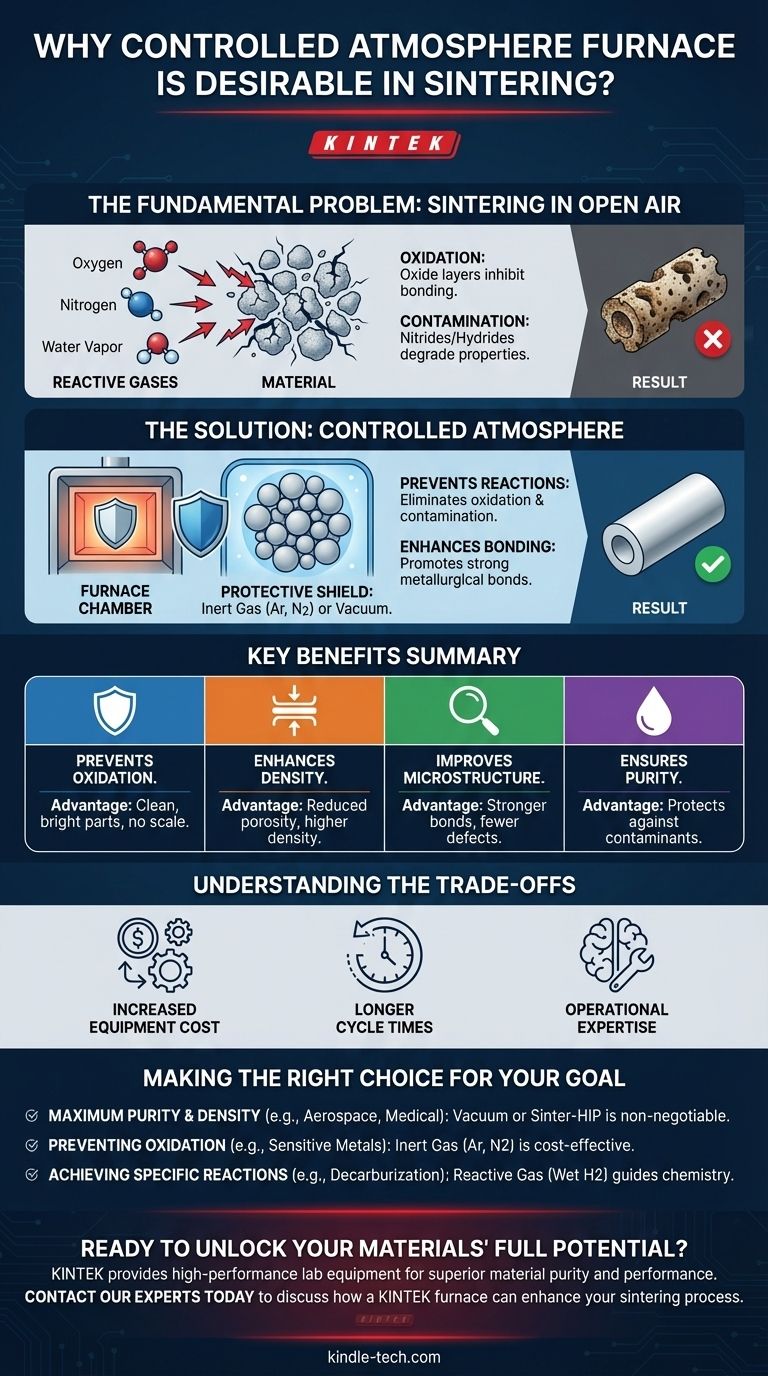
The Fundamental Problem: Sintering in Open Air
To understand the value of a controlled atmosphere, we must first recognize the inherent problems of sintering in a standard, ambient environment.
The Challenge of Reactive Gases
Normal air is approximately 78% nitrogen and 21% oxygen, with traces of water vapor and other gases. At the high temperatures required for sintering, these gases are no longer passive bystanders; they become highly reactive chemical agents.
The Formation of Unwanted Oxides
The most common issue is oxidation. Oxygen readily bonds with the surfaces of metal or ceramic particles, forming oxide layers. These layers act as a barrier, physically inhibiting the particle-to-particle bonding that is the entire purpose of sintering.
The Impact on Material Purity
Beyond oxygen, elements like nitrogen and hydrogen (from water vapor) can also react with the material. This can lead to the formation of nitrides or hydrides, which are considered contaminants that degrade the final material's intended mechanical, electrical, or magnetic properties.
How a Controlled Atmosphere Solves These Problems
By replacing the reactive ambient air with a carefully managed environment, a controlled atmosphere furnace directly counteracts these issues, unlocking a new level of material quality.
Preventing Chemical Degradation
The primary benefit is the prevention of adverse chemical reactions. By removing oxygen, you eliminate oxidation, resulting in clean, bright parts without a surface scale. By removing other gases, you prevent issues like decarburization or unwanted nitriding, ensuring the material's chemistry remains exactly as designed.
Enhancing Material Density
A vacuum environment is particularly effective at improving densification. As the furnace is evacuated, it pulls residual gas out from within the pores of the compacted powder. This removal of trapped gas allows the pores to collapse more completely during sintering, leading to a higher final density and reduced porosity.
Improving Microstructure and Bonding
A controlled atmosphere, especially a vacuum, can actively remove pre-existing oxide films from the powder particles before the sintering temperature is reached. This cleans the particle surfaces, dramatically improving wettability and promoting direct, strong metallurgical bonds between particles for a more robust microstructure.
Achieving Superior Final Properties
The culmination of these benefits is a final product with demonstrably superior qualities. Materials sintered in a controlled atmosphere consistently exhibit higher strength, greater wear resistance, and fewer internal defects, making the process essential for high-performance applications in aerospace, medical, and electronics industries.
Understanding the Trade-offs
While highly effective, adopting a controlled atmosphere process involves considering its inherent complexities.
Increased Equipment Cost
Controlled atmosphere and vacuum furnaces are significantly more complex than simple air furnaces. They require robust sealing, vacuum pumps, gas management systems, and sophisticated controls, all of which lead to a higher initial capital investment.
Longer Cycle Times
Achieving the desired atmosphere is not instantaneous. Pumping down a chamber to a deep vacuum or purging it completely with an inert gas adds considerable time to the overall process cycle compared to simply heating a part in air.
Operational Expertise
Properly operating and maintaining these systems requires a higher level of technical skill. Operators must understand vacuum technology, gas handling, and safety protocols to ensure both product quality and safe operation.
Making the Right Choice for Your Goal
The decision to use a controlled atmosphere depends entirely on the requirements of your final component.
- If your primary focus is maximum purity and density for advanced materials (e.g., aerospace superalloys, medical implants): A vacuum or Sinter-HIP furnace is non-negotiable to eliminate all atmospheric contamination.
- If your primary focus is preventing oxidation on sensitive metals without requiring a full vacuum: An inert gas atmosphere (like argon or nitrogen) provides a cost-effective and highly effective protective shield.
- If your primary focus is achieving specific chemical reactions (e.g., removing carbon): A reactive gas atmosphere (like wet hydrogen) can be used to actively participate in and guide the material's chemistry.
Ultimately, controlling the atmosphere transforms sintering from a simple heating process into a precise manufacturing tool.
Summary Table:
| Benefit | Key Advantage |
|---|---|
| Prevents Oxidation | Eliminates oxide layers for clean, bright parts. |
| Enhances Density | Removes trapped gases for reduced porosity. |
| Improves Microstructure | Cleans particle surfaces for stronger bonds. |
| Ensures Purity | Protects against contaminants like nitrides. |
Ready to unlock the full potential of your materials?
For laboratory professionals demanding superior material purity and performance, a controlled atmosphere furnace is essential. KINTEK specializes in high-performance lab equipment, including controlled atmosphere furnaces designed to meet the rigorous demands of sintering advanced ceramics, metals, and alloys.
We provide the tools to eliminate contamination and achieve the high-density, defect-free components required in aerospace, medical, and electronics manufacturing.
Contact our experts today to discuss how a KINTEK furnace can enhance your sintering process and deliver consistent, high-quality results.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Spark Plasma Sintering Furnace SPS Furnace
People Also Ask
- Can nitrogen be used for brazing? Key Conditions and Applications Explained
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- What is an inert condition? A Guide to Preventing Fires and Explosions
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes



















