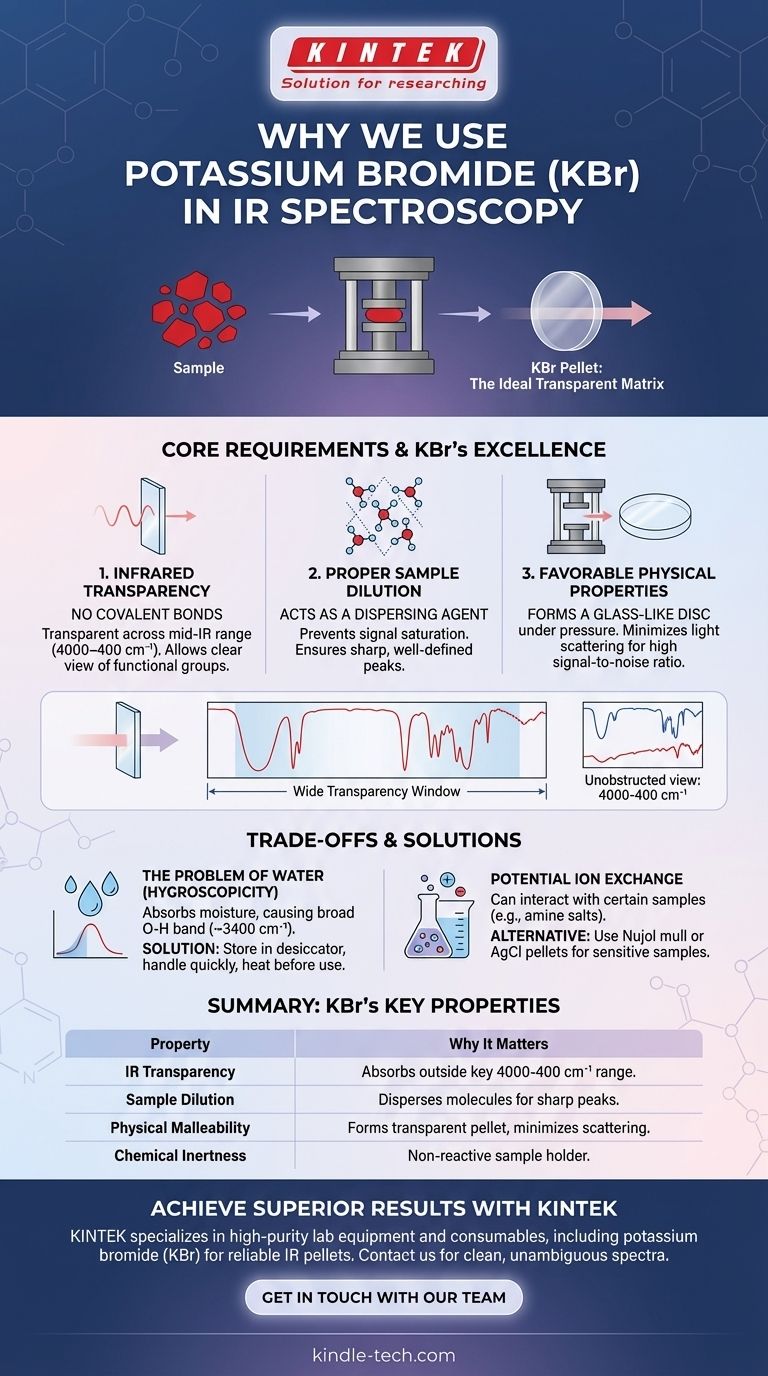
In short, we use potassium bromide (KBr) in IR spectroscopy because it is transparent to infrared radiation across the most useful frequency range. It also possesses the ideal physical properties to be pressed into a thin, uniform pellet that can hold a solid sample for analysis without interfering with the measurement.
The core challenge in analyzing solid samples with IR spectroscopy is preparing them in a way that allows light to pass through without being scattered or absorbed by the holder material. KBr serves as an ideal solid-state "solvent," creating a transparent matrix that isolates the sample molecules and allows for a clean, unobstructed spectrum.

The Core Requirements of a Sample Matrix
To obtain a high-quality infrared spectrum of a solid, the material used to hold the sample—the matrix—must meet several strict criteria. The choice of matrix is as important as the spectrometer itself.
Requirement 1: Infrared Transparency
The fundamental principle of absorption spectroscopy is to measure the light absorbed by your sample, not the container.
An ideal matrix must not have molecular bonds that vibrate (and thus absorb) in the same energy range as the sample. KBr, being a simple ionic salt (K⁺Br⁻), has no covalent bonds. Its ionic lattice vibrations occur at very low frequencies, well outside the standard mid-IR range (4000–400 cm⁻¹) where we identify functional groups.
Requirement 2: Proper Sample Dilution
Just as in liquid-state analysis, a sample that is too concentrated will produce a poor spectrum.
High concentrations cause peaks to become overly broad and flat-topped (a phenomenon called signal saturation), making them impossible to interpret. KBr powder acts as a dispersing agent, separating the sample molecules from each other within the pellet. This ensures the IR beam interacts with isolated molecules, producing sharp, well-defined peaks.
Requirement 3: Favorable Physical Properties
The matrix must form a solid disc that is clear, not cloudy. Any cloudiness or imperfection will scatter the IR beam, reducing the signal-to-noise ratio and distorting the baseline of the spectrum.
KBr is a relatively soft, crystalline material. Under high pressure, its crystal structure flows and deforms to create a homogenous, transparent, glass-like disc. Harder materials would fracture or fail to fuse properly, resulting in an opaque pellet that scatters light.
Why Potassium Bromide (KBr) Excels
KBr meets all the primary requirements for a solid-state IR matrix, making it the default choice for this technique.
A Wide Window of Transparency
KBr is transparent from the far-UV all the way through the mid-IR, with a cutoff around 400 cm⁻¹. This provides an unobstructed view of the entire functional group region (4000-1500 cm⁻¹) and the complex "fingerprint region" (1500-400 cm⁻¹), which is critical for identifying compounds.
Ideal Malleability for Pellets
The softness of KBr allows it to be ground into a fine powder with the sample, ensuring a homogenous mixture. When pressed in a die, it fuses into a solid pellet with minimal light scattering, maximizing the quality of the spectrum.
General Chemical Inertness
For the vast majority of organic and inorganic compounds, KBr is non-reactive. It simply serves as an inert matrix to hold the sample in the path of the IR beam.
Understanding the Trade-offs and Common Pitfalls
While KBr is the standard, it is not without limitations. Understanding these is key to avoiding common errors and correctly interpreting your results.
The Problem of Water: Hygroscopicity
The most significant drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
This water will appear in your spectrum, creating a very broad absorption band centered around 3400 cm⁻¹ (O-H stretching) and a smaller, sharper band around 1640 cm⁻¹ (H-O-H bending). To avoid this, KBr must be stored in a desiccator and handled quickly. Often, heating the KBr in an oven before use is necessary to drive off any absorbed water.
Potential for Ion Exchange
Because KBr is an ionic salt, it can sometimes interact with certain types of samples. For example, when analyzing the hydrochloride salt of an amine (R-NH₃⁺Cl⁻), the bromide from the KBr pellet can sometimes exchange with the chloride, altering the sample's true structure and spectrum.
When Not to Use a KBr Pellet
If a sample is highly sensitive to moisture, cannot be ground into a fine powder, or reacts with KBr, an alternative method is needed. The most common alternative is a Nujol mull, where the solid is ground with a mineral oil (Nujol) to form a paste. However, this method introduces interfering peaks from the oil itself.
Making the Right Choice for Your Goal
Selecting the correct sample preparation technique is crucial for obtaining reliable data.
- If your primary focus is a general-purpose solid sample: A KBr pellet is the gold standard for a high-quality, full-range spectrum, provided you handle the material correctly to avoid water contamination.
- If your sample is sensitive to moisture or pressure: Consider preparing a Nujol mull, but be prepared to subtract or ignore the known C-H absorption bands from the mineral oil.
- If your sample is an ionic salt prone to halide exchange: Be cautious with KBr. A Nujol mull or specialized pellets made from silver chloride (AgCl) may be a more reliable choice.
Ultimately, understanding the properties of your sample matrix is the first step toward acquiring a clean and trustworthy infrared spectrum.
Summary Table:
| Property | Why It Matters for IR Spectroscopy |
|---|---|
| IR Transparency | No covalent bonds; absorbs outside the key 4000-400 cm⁻¹ range, allowing a clear view of your sample's spectrum. |
| Sample Dilution | Disperses sample molecules to prevent signal saturation, ensuring sharp, well-defined peaks for accurate analysis. |
| Physical Malleability | Presses into a hard, transparent pellet that minimizes light scattering, maximizing signal-to-noise ratio. |
| Chemical Inertness | Generally non-reactive with most organic and inorganic compounds, serving as a passive sample holder. |
Ready to achieve superior results in your lab?
KINTEK specializes in high-purity lab equipment and consumables, including potassium bromide (KBr) perfect for creating reliable IR pellets. Our products are designed to help you obtain clean, unambiguous spectra for your solid samples, ensuring the accuracy of your research and quality control.
Contact us today to discuss your laboratory needs and let our experts help you select the right materials for precise and efficient spectroscopy.
Get in touch with our team now!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- What is the mechanical function of an overhead digital stirrer? Optimize Molybdenum Disk Dissolution Efficiency
- How should steel be heated during heat treatment? Master the 3 Stages for Perfect Results
- What is the difference between batch furnace and continuous furnace? Choose the Right System for Your Production Volume
- Why is high-power ultrasound utilized for MOFs in MMMs? Unlock Superior Gas Separation & Uniform Dispersion
- How does laboratory drying equipment affect the performance of hydrogel carriers? Optimize Drug Loading and Release
- Can you harden non-ferrous metals? Yes, with the right methods for aluminum, copper, and titanium
- What is the HIP process in powder metallurgy? Achieve Full Density & Superior Material Properties
- What is titanium disadvantages and advantages? Weighing Performance vs. Cost for Your Project



















