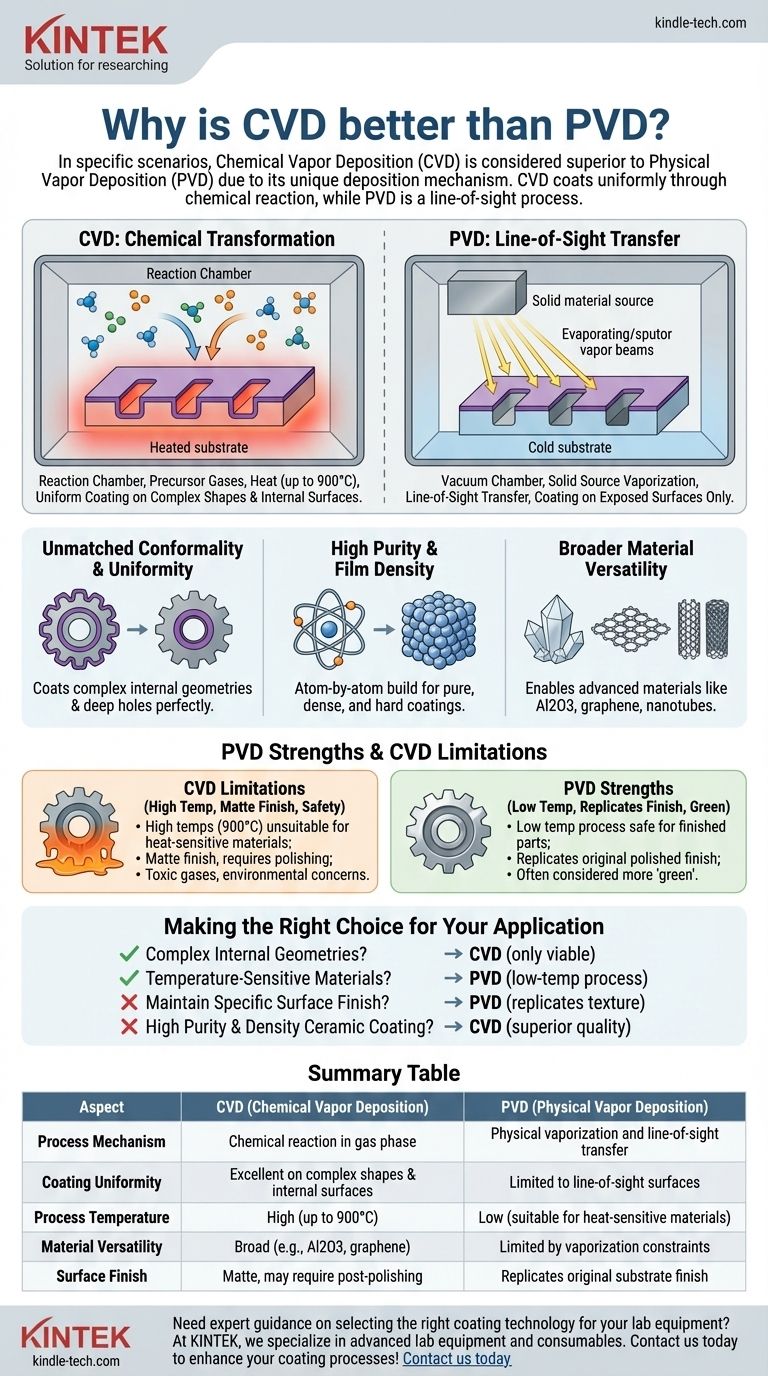
In specific scenarios, Chemical Vapor Deposition (CVD) is considered superior to Physical Vapor Deposition (PVD) due to its unique deposition mechanism. Unlike PVD, which is a line-of-sight process, CVD uses a chemical reaction to deposit a film. This fundamental difference allows it to uniformly coat complex shapes and internal surfaces that are impossible to reach with PVD, while also creating exceptionally pure and dense coatings from a wide range of materials.
The question isn't whether CVD is universally 'better' than PVD, but which process is fundamentally suited to your material, geometry, and performance goals. CVD's strength lies in its ability to coat everything it touches through a chemical reaction, while PVD excels in low-temperature applications on line-of-sight surfaces.

The Fundamental Difference: Chemical vs. Physical
To understand why one method is chosen over the other, you must first grasp how they operate. Their names describe their core processes perfectly.
How CVD Works: A Chemical Transformation
Chemical Vapor Deposition involves introducing volatile precursor gases into a reaction chamber containing the part to be coated, known as the substrate.
The substrate is heated to a very high temperature (often up to 900°C), which triggers a chemical reaction in the gases. This reaction causes a new, solid material to form and deposit as a thin, uniform film on the heated substrate's surface.
How PVD Works: A Line-of-Sight Transfer
Physical Vapor Deposition, in contrast, works by physically transforming a solid coating material into a vapor. This is typically done through processes like sputtering or evaporation in a vacuum chamber.
This vapor then travels in a straight line—like a beam of light—and condenses onto the substrate, forming the coating. Any surface not in the direct line of sight of the vapor source will not be coated.
Key Advantages of the CVD Process
The chemical nature of CVD gives it distinct advantages in certain applications.
Unmatched Conformality and Uniformity
Because the precursor gases surround the entire component, the chemical reaction and subsequent deposition happen on every exposed surface. This means CVD can create a perfectly uniform coating inside deep holes, around sharp corners, and on highly complex geometries.
PVD, being line-of-sight, cannot achieve this. It's much like trying to spray paint the inside of a long, narrow tube—only the entrance will get coated.
High Purity and Film Density
The CVD process builds the coating layer atom-by-atom through a controlled chemical reaction. This results in films that are exceptionally pure, dense, and fine-grained.
These characteristics often lead to superior hardness and wear resistance compared to coatings made through other methods.
Broader Material Versatility
CVD can create coatings from elements that are very difficult to evaporate or sputter with PVD. For example, creating high-performance aluminum oxide (Al2O3) coatings, known for their incredible hardness and stability, is a classic strength of the CVD process.
It can also be used to produce advanced materials like large-scale graphene sheets and carbon nanotube arrays, which are not feasible with PVD.
Understanding the Trade-offs: Why CVD Isn't Always the Answer
CVD's strengths come with significant limitations that make PVD the better, or only, choice in many common industrial applications.
High Process Temperatures
The primary drawback of CVD is the extreme heat required. Temperatures of 900°C will ruin the temper of heat-treated steels and are far too high for many other metals, alloys, and plastics. This single factor disqualifies CVD for a vast range of applications.
PVD, conversely, operates at much lower temperatures, making it safe for coating finished, heat-sensitive parts without altering their underlying material properties.
Surface Finish Alterations
The CVD process typically results in a matte, non-reflective surface finish. If a part requires a polished or decorative appearance, it must undergo a secondary polishing step after coating.
PVD has the distinct advantage of replicating the substrate's original surface finish. A part that goes into the PVD chamber polished will come out with a polished, colored coating.
Environmental and Safety Concerns
The precursor gases used in many CVD processes can be toxic, corrosive, or flammable, requiring specialized handling and exhaust management systems. PVD is often considered a more environmentally friendly "green" process.
Making the Right Choice for Your Application
Choosing between CVD and PVD requires a clear analysis of your project's specific constraints and objectives.
- If your primary focus is coating complex internal geometries or non-line-of-sight surfaces: CVD is the only viable technology for achieving a uniform film.
- If your primary focus is coating temperature-sensitive materials (like hardened tool steel or aluminum): PVD is the clear and necessary choice due to its low-temperature process.
- If your primary focus is maintaining a specific surface finish (e.g., polished or decorative): PVD is superior as it directly replicates the original texture of the part.
- If your primary focus is creating a highly pure, dense ceramic coating like Al2O3: CVD often provides a higher quality and more stable film.
Ultimately, selecting the right coating technology depends on a clear understanding of your material's limitations and your component's final application.
Summary Table:
| Aspect | CVD (Chemical Vapor Deposition) | PVD (Physical Vapor Deposition) |
|---|---|---|
| Process Mechanism | Chemical reaction in gas phase | Physical vaporization and line-of-sight transfer |
| Coating Uniformity | Excellent on complex shapes and internal surfaces | Limited to line-of-sight surfaces |
| Process Temperature | High (up to 900°C) | Low (suitable for heat-sensitive materials) |
| Material Versatility | Broad (e.g., Al2O3, graphene) | Limited by vaporization constraints |
| Surface Finish | Matte, may require post-polishing | Replicates original substrate finish |
Need expert guidance on selecting the right coating technology for your lab equipment? At KINTEK, we specialize in providing advanced lab equipment and consumables tailored to your specific research and production needs. Whether you're working with complex geometries or temperature-sensitive materials, our team can help you choose the optimal solution for superior performance. Contact us today to discuss how our expertise can enhance your coating processes!
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the difference between CVD and PECVD? Choose the Right Thin-Film Deposition Method
- What is the difference between thermal CVD and PECVD? Choose the Right Thin-Film Deposition Method
- What are the examples of CVD method? Discover the Versatile Applications of Chemical Vapor Deposition
- What is the difference between CVD and PVD process? A Guide to Choosing the Right Coating Method
- How is PECVD different from CVD? Unlock Low-Temperature Thin Film Deposition



















