At its core, Graphite Furnace Atomic Absorption (GFAAS) is more sensitive than Flame Atomic Absorption (FAAS) because it confines the entire sample's atoms in the instrument's light path for a much longer time. This extended residence time, combined with superior atomization efficiency in a controlled environment, allows the instrument to detect a much stronger signal from the same amount of an element.
The fundamental difference is not merely the heat source, but how each technique handles the sample. A graphite furnace atomizes a discrete, contained sample, creating a dense cloud of atoms with a long lifetime. A flame, by contrast, continuously and inefficiently atomizes a flowing sample that rushes past the detector in milliseconds.

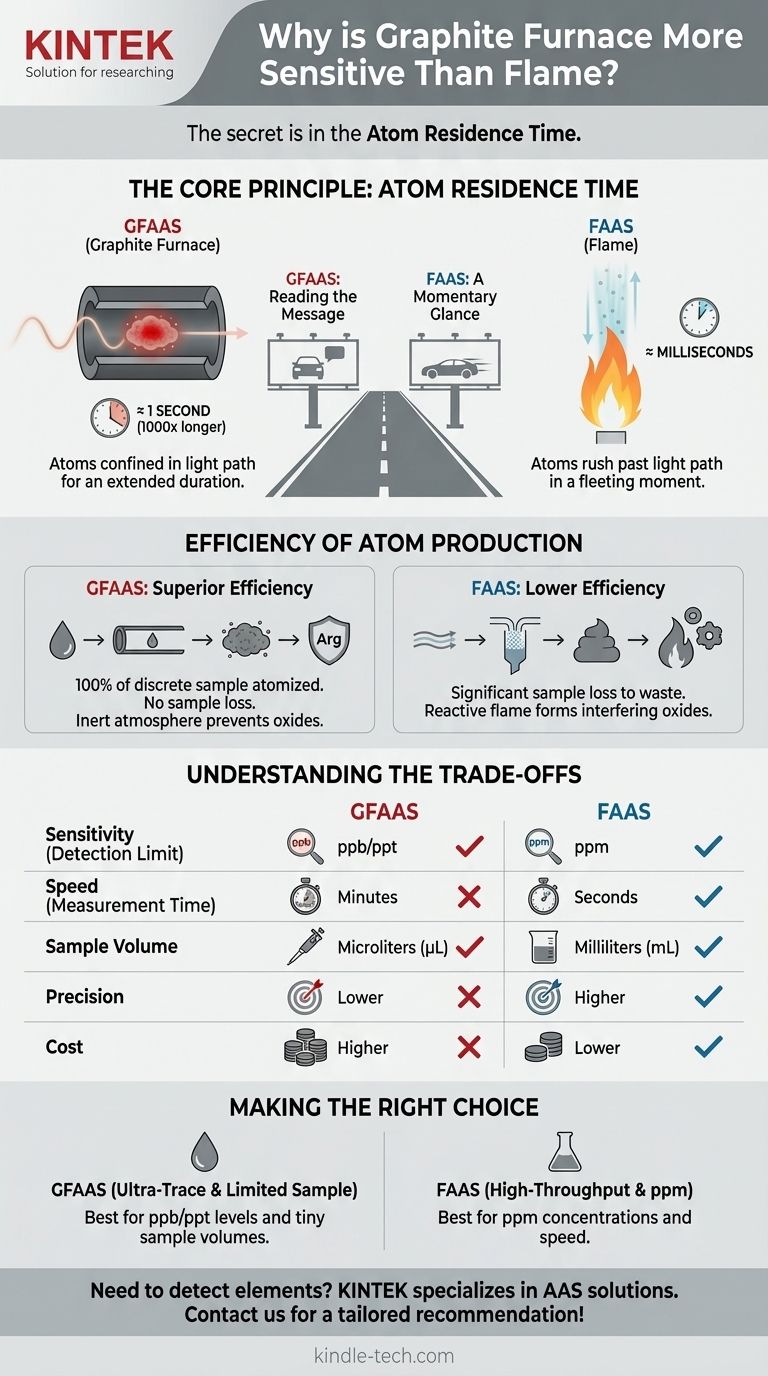
The Core Principle: Atom Residence Time
The single most important factor driving the sensitivity difference is residence time—the average duration an atom spends in the light beam where it can absorb energy.
The Fleeting Moment in a Flame (FAAS)
In flame AA, the sample is continuously aspirated into the flame. The high-velocity gases of the flame rush the newly created atoms up and out of the light path.
An individual atom's time in the light path is incredibly brief, typically on the order of milliseconds. This gives each atom only a tiny window of opportunity to absorb light.
The Confined Cloud in a Furnace (GFAAS)
In a graphite furnace, a small, discrete volume of the sample is placed inside a graphite tube. The tube is then sealed and heated in a programmed sequence.
When the final, high-temperature atomization step occurs, the resulting cloud of atoms is trapped within the confines of the tube. These atoms remain in the light path for one or more seconds—a thousand-fold increase over the flame.
Analogy: The Billboard on the Highway
Imagine the atoms are people and the instrument's light beam is a billboard you need them to read.
- FAAS is like having the people drive past the billboard on a high-speed freeway. Each person gets only a momentary glance.
- GFAAS is like having the same people stop their cars, get out, and stand directly in front of the billboard for several seconds. The chance of them reading and understanding the message is vastly higher.
Efficiency of Atom Production
Beyond just residence time, the entire process of converting a sample into free, ground-state atoms is far more efficient in a graphite furnace.
Atomization Efficiency
In FAAS, much of the aspirated sample goes directly to waste and never even reaches the flame. The nebulization process is also inherently inefficient.
In GFAAS, 100% of the discrete sample placed in the tube is subjected to the heating program and atomized. There is no sample loss during introduction, leading to a much higher concentration of atoms from a given starting material.
Sample Volume and Atom Density
FAAS requires a continuous flow of sample, effectively diluting the analyte in a large volume of oxidant and fuel gases. The resulting atomic cloud is diffuse.
GFAAS atomizes a tiny microliter volume into a very small, enclosed space. This creates a transient but extremely dense cloud of atoms, maximizing the absorption signal.
The Chemical Environment
A flame is a highly reactive, oxidizing environment. This can cause the target atoms to form stable oxides that do not absorb light at the desired wavelength, further reducing the signal.
A graphite furnace is continuously purged with an inert gas (typically argon). This protective atmosphere prevents the formation of oxides, ensuring the atoms remain in their elemental, light-absorbing state for a longer period.
Understanding the Trade-offs
The superior sensitivity of GFAAS comes with significant practical and analytical trade-offs. It is not always the better technique.
Speed vs. Sensitivity
A single FAAS measurement takes a few seconds. A single GFAAS measurement, with its required drying, charring, atomization, and clean-out steps, takes several minutes. For analyses where concentration is high and throughput is key, FAAS is vastly superior.
Matrix Interferences
GFAAS is far more susceptible to background absorption and chemical interferences from the sample matrix. This necessitates more complex and powerful background correction systems (like Zeeman correction) and more intensive method development.
Precision and Cost
The steady-state signal of FAAS often provides better precision (lower relative standard deviation) than the transient, peak-shaped signal of GFAAS. Furthermore, GFAAS instruments and their consumable graphite tubes are significantly more expensive to purchase and operate.
Making the Right Choice for Your Analysis
Choosing between FAAS and GFAAS requires a clear understanding of your analytical goals.
- If your primary focus is high throughput for concentrations in the ppm (mg/L) range: FAAS is the clear choice for its speed, simplicity, and excellent precision.
- If your primary focus is detecting ultra-trace levels in the ppb (µg/L) or ppt (ng/L) range: GFAAS is required, as FAAS lacks the necessary sensitivity.
- If your sample volume is extremely limited: GFAAS is the only option, as it can perform an analysis on just a few microliters of sample.
- If you are analyzing samples with a simple, clean matrix and require good precision: FAAS is often the more robust and reliable method.
By understanding these fundamental differences in atom confinement and efficiency, you can confidently select the precise tool for your analytical challenge.
Summary Table:
| Feature | Graphite Furnace AA (GFAAS) | Flame AA (FAAS) |
|---|---|---|
| Detection Limit | Parts per billion (ppb) / trillion (ppt) | Parts per million (ppm) |
| Atom Residence Time | ~1 second (confined in tube) | ~Milliseconds (flushed through flame) |
| Sample Volume | Microliters (µL) | Milliliters (mL) |
| Best For | Ultra-trace analysis, limited samples | High-throughput, higher concentration analysis |
Need to detect elements at ultra-trace levels? KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the right atomic absorption spectroscopy solution—whether it's a high-sensitivity graphite furnace system for ppb detection or a high-throughput flame system for routine analysis. Contact us today to discuss your application and get a tailored recommendation!
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- Why is sieve analysis important? Ensure Consistent Quality and Performance of Your Materials
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis
- What is the primary function of a mechanical sieve shaker for biomass analysis? Optimize Particle Size Distribution
- How are vibratory sieve shakers and standard sieves utilized to analyze the effects of biomass torrefaction? Optimize Grindability
- What are the factors affecting sieving performance and efficiency? Optimize Your Particle Separation Process



















