Properties and Characteristics of Zirconia
Physical and Chemical Properties
Zirconia (ZrO₂) is renowned for its exceptional physical and chemical properties, which make it a material of choice in numerous industrial applications. One of its most notable characteristics is its high melting and boiling points, which significantly exceed those of many other ceramics. This thermal stability allows ZrO₂ to withstand extreme temperatures, making it an ideal refractory material for processes such as glass melting and steel smelting.
In addition to its thermal resilience, ZrO₂ exhibits high hardness, comparable to that of sapphire and silicon carbide. This hardness endows zirconia with excellent wear resistance, which is crucial for applications where durability is paramount. At room temperature, zirconia behaves as an insulator, meaning it does not conduct electricity. However, as the temperature rises, it undergoes a transition to become a conductor, a property that is leveraged in certain high-temperature electronic devices.
| Property | Value/Description |
|---|---|
| Melting Point | ~2715°C |
| Boiling Point | ~4682°C |
| Hardness (Mohs scale) | ~8.5 |
| Electrical Conductivity | Insulator at room temperature, conductor at high temperatures |
| Thermal Conductivity | Low, making it useful for thermal barrier coatings |
The combination of these properties has led to the extensive use of zirconia in refractory materials since the 1920s. Its ability to maintain structural integrity under high temperatures has made it indispensable in industries that require materials to withstand extreme conditions.

Crystal Forms and Transformations
Pure zirconia exhibits three distinct crystal forms under normal atmospheric pressure, each characterized by its unique structural arrangement and temperature range. At low temperatures, zirconia crystallizes in the monoclinic system, a form that is relatively stable but susceptible to transformation under thermal stress. As the temperature increases, zirconia undergoes a phase transition to the tetragonal system, a crystal structure that is more stable at moderate temperatures but still prone to further transformation. At even higher temperatures, zirconia adopts the cubic system, which is the most thermodynamically stable form under these conditions.
The transformation between these crystal forms is not merely a structural change; it is accompanied by significant volume changes. This phenomenon, known as the martensitic transformation, can lead to substantial internal stresses within the material. When zirconia transitions from the monoclinic to the tetragonal form, or from the tetragonal to the cubic form, the resulting volume changes can induce cracks if the material is not adequately stabilized. This instability is a critical consideration in the fabrication and application of zirconia ceramics, as it affects both the mechanical integrity and the overall performance of the material.
To mitigate the risk of cracking during these transformations, various stabilization techniques have been developed. These techniques often involve the addition of dopants, such as yttrium oxide (Y₂O₃) or calcium oxide (CaO), which help to stabilize the tetragonal or cubic phases at lower temperatures. By controlling the composition and microstructure of zirconia, manufacturers can tailor the material's properties to meet specific performance requirements, thereby expanding its applications across a wide range of industries.
Development and History of Zirconia Ceramics
Early Discoveries and Industrial Applications
The journey of zirconium oxide from its early extraction to its industrial applications is a testament to human ingenuity and technological progress. As early as 1789, Martin Heinrich Klaproth, a German chemist, successfully isolated zirconium oxide from gemstones, marking a significant milestone in the exploration of this material. However, it took several decades for zirconium oxide to find its footing in industrial applications.

The 1940s saw the first significant industrial use of zirconium oxide, particularly in the form of gas lampshades. This application highlighted its unique properties, such as high melting points and excellent thermal stability, which made it ideal for withstanding the high temperatures required in lighting technology.
Since then, zirconium oxide has expanded its role in various industrial sectors. One of its most notable uses is in refractory materials, where its ability to withstand extreme temperatures without degrading is invaluable. This property has made it an essential component in the production of glass and steel, where it serves as a protective lining in furnaces and other high-temperature environments.
In addition to its use in refractory materials, zirconium oxide has found applications in coloring and abrasives. Its versatility and durability make it a preferred choice for these purposes, contributing to the development of high-performance coatings and abrasive tools.
| Application | Description |
|---|---|
| Gas Lampshades | Initial industrial use in the 1940s, leveraging high melting points and thermal stability. |
| Refractory Materials | Essential in glass and steel production, providing protection against extreme temperatures. |
| Coloring | Used in high-performance coatings, enhancing durability and aesthetics. |
| Abrasives | Preferred for abrasive tools due to its hardness and wear resistance. |
The early discoveries and subsequent industrial applications of zirconium oxide underscore its potential and versatility, paving the way for more advanced uses in modern technology.
Modern Research and Advancements
Since 1975, the field of zirconia ceramics has seen significant advancements, largely due to the pioneering work of Australian scholar K.C. Ganvil. Ganvil introduced a novel concept that harnessed the volume effect generated by ZrO2 phase transitions to enhance the toughness of ceramics. This groundbreaking idea sparked a wave of research and innovation, transforming zirconia ceramics from a niche material into a structural powerhouse.
The phase transition of zirconia, which involves transformations between monoclinic, tetragonal, and cubic crystal systems, presents both challenges and opportunities. While these transitions can lead to cracking due to volume changes, Ganvil's approach turned this liability into an asset by strategically controlling these transitions to improve the material's durability and strength. This innovative approach has not only expanded the application scope of zirconia ceramics but also set new standards for material science research.
Over the years, researchers have built upon Ganvil's foundational work, developing sophisticated methods to manipulate these phase transitions. Techniques such as thermal treatments, doping with stabilizers, and advanced sintering processes have been employed to optimize the mechanical properties of zirconia ceramics. These advancements have paved the way for the material's use in high-stress environments, such as aerospace components, medical implants, and cutting-edge electronics.
The active research in zirconia ceramics has also led to the development of new applications that were previously unimaginable. For instance, the material's biocompatibility and mechanical strength make it an ideal candidate for dental and orthopedic implants. Additionally, its thermal stability and resistance to corrosion have opened up new avenues in the chemical and energy sectors.
In summary, the modern research and advancements in zirconia ceramics, catalyzed by Ganvil's pioneering concept, have propelled the material into the forefront of structural applications. This ongoing innovation continues to push the boundaries of what zirconia ceramics can achieve, promising even more exciting developments in the future.
Preparation Methods of Zirconia Powder
Physical Methods
Physical methods for preparing zirconia powder primarily encompass mechanical pulverization and vacuum freeze drying. These techniques are relatively straightforward and cost-effective, making them popular choices in various industrial applications. However, they are not without their drawbacks.
One of the most significant challenges associated with mechanical pulverization is the issue of non-uniform particle size distribution. This heterogeneity can lead to inconsistencies in the final product, which may affect its performance and reliability. Additionally, the process of mechanical pulverization can introduce contaminants into the powder, which can compromise the purity and integrity of the zirconia material.
Vacuum freeze drying, while effective in preserving the integrity of the material, also faces limitations. This method is often used to prevent the formation of large agglomerates, which can be problematic in subsequent processing steps. Despite its advantages, vacuum freeze drying can be a time-consuming and energy-intensive process, which may not be feasible for large-scale production.
In summary, while physical methods offer simplicity and affordability, they require careful consideration to mitigate issues related to particle size uniformity and contamination.
Chemical Methods
Chemical methods for preparing zirconia powder are pivotal in achieving high purity and controlled particle size, which are critical for the material's performance in various applications. These methods include co-precipitation, hydrothermal synthesis, sol-gel processing, high-temperature spray pyrolysis, and chemical vapor deposition (CVD). Each technique offers unique advantages and challenges, contributing to the versatility of zirconia ceramics.
Co-precipitation
Co-precipitation involves the simultaneous precipitation of zirconia and other metal oxides from a solution. This method is particularly useful for creating complex oxide materials with tailored properties. However, it requires precise control over pH, temperature, and reaction time to ensure uniform particle distribution and avoid agglomeration.
Hydrothermal Synthesis
Hydrothermal synthesis utilizes high-pressure and high-temperature water to facilitate the formation of zirconia particles. This technique is advantageous for producing nanoscale zirconia with high crystallinity and uniform morphology. The main limitation is the need for specialized equipment that can withstand high pressures and temperatures, making it cost-prohibitive for some applications.
Sol-Gel Processing
Sol-gel processing involves the conversion of a colloidal suspension (sol) into a gel, which is then dried and calcined to form zirconia particles. This method allows for precise control over particle size and morphology, making it ideal for creating high-purity, ultrafine zirconia powders. However, the lengthy process and the potential for residual organic content can be drawbacks.
High-Temperature Spray Pyrolysis
High-temperature spray pyrolysis involves spraying a precursor solution into a hot zone where it undergoes rapid pyrolysis to form zirconia particles. This technique is known for its ability to produce particles with narrow size distributions and high purity. The major limitation is the complexity of the equipment and the high energy consumption required for the process.
Chemical Vapor Deposition (CVD)
Chemical vapor deposition involves the reaction of precursor gases to form zirconia particles on a substrate. This method is particularly useful for creating thin films and coatings with exceptional purity and controlled thickness. However, the equipment and operational costs are high, limiting its widespread use.
These chemical methods collectively provide a robust toolkit for the synthesis of zirconia powders, each addressing specific needs in terms of purity, particle size, and morphology. Despite their limitations, these techniques continue to advance, driven by the demand for high-performance zirconia ceramics in diverse industries.
Molding and Sintering Processes
Molding Techniques
The molding of zirconia ceramics involves several sophisticated techniques, each tailored to specific applications and challenges. These techniques include dry pressing, isostatic pressing, hot die casting, slip injection molding, and tape casting. Each method offers unique advantages and limitations, making them suitable for different production scenarios.
Dry Pressing is a common technique where zirconia powder is compacted under high pressure in a die. This method is particularly advantageous for its simplicity and efficiency, making it suitable for mass production of simple shapes. However, it can result in non-uniform density and requires careful control of the pressing parameters to avoid defects.
Isostatic Pressing involves applying equal pressure from all directions to the zirconia powder, ensuring uniform density and shape. This technique is ideal for complex geometries and can produce high-quality parts with minimal internal stresses. The main challenge lies in the equipment's complexity and cost.
Hot Die Casting is used for creating intricate shapes by injecting molten zirconia into a mold at high temperatures. This method allows for the production of detailed and precise components, but it requires precise temperature control and can be energy-intensive.
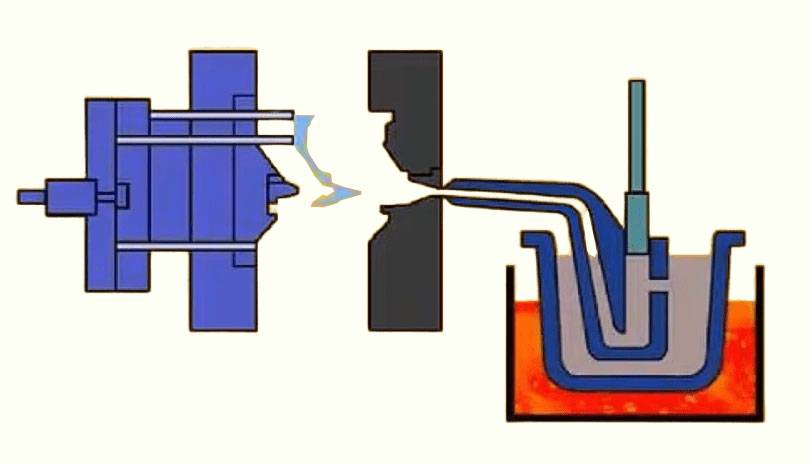
Slip Injection Molding involves mixing zirconia powder with a liquid medium to form a slurry, which is then injected into a mold. This technique is particularly useful for producing thin-walled and complex shapes. However, it requires careful control of the slurry's viscosity and drying conditions to prevent cracking.
Tape Casting, also known as doctor blade or slip casting, is a method where a slurry of zirconia powder is spread into a thin layer and then dried to form a flexible tape. This technique is widely used in the production of multilayer ceramics and electronic components. The main challenge is achieving uniform thickness and avoiding defects during the drying process.
Each of these molding techniques plays a crucial role in the production of zirconia ceramics, contributing to the material's versatility and wide-ranging applications.
Sintering Methods
Sintering techniques are crucial for transforming zirconia powder into high-density, mechanically robust ceramic components. The primary methods include conventional sintering, hot pressing sintering, hot isostatic pressing sintering, and microwave sintering. Each technique offers distinct advantages tailored to specific applications.
Conventional Sintering is the most straightforward method, involving the heating of a compacted powder to the appropriate temperature without external pressure. This technique can be executed in box or tube furnaces, but it necessitates a controlled atmosphere to ensure both safety and optimal results. While simple, conventional sintering may not yield the highest densities or the most uniform properties.
Hot Pressing Sintering and Hot Isostatic Pressing Sintering both apply pressure during the sintering process, which significantly enhances the density and homogeneity of the final product. Hot pressing involves applying pressure uniaxially, while hot isostatic pressing uses a gas to apply pressure uniformly from all directions. These methods are particularly beneficial for achieving near-theoretical densities and minimizing porosity.
Microwave Sintering represents a more innovative approach, leveraging microwave energy to heat the material internally. This method can result in faster sintering times and potentially lower energy consumption compared to traditional techniques. However, it requires specific conditions and materials that are sensitive to microwave heating.
The choice of sintering method depends on the desired properties of the final product, the specific application, and the constraints of the production process. Each technique has its own set of advantages and challenges, making it essential to carefully select the most appropriate method for a given application.
| Sintering Method | Advantages | Challenges |
|---|---|---|
| Conventional Sintering | Simple, widely applicable | May not achieve highest densities, requires controlled atmosphere |
| Hot Pressing Sintering | High density, uniform properties | Requires specialized equipment, can be costly |
| Hot Isostatic Pressing Sintering | Near-theoretical density, excellent homogeneity | Complex and expensive, requires precise control of gas pressure |
| Microwave Sintering | Faster sintering times, potential energy savings | Requires materials sensitive to microwave heating, specific conditions |
Understanding these sintering methods and their implications is vital for achieving the desired properties in zirconia ceramics, ensuring their performance in various industrial and commercial applications.
Applications of Zirconia Ceramics
Industrial and Commercial Uses
Zirconia ceramics have found extensive applications across a multitude of industries, each leveraging its unique properties to enhance performance and durability. In the realm of 3C electronics, zirconia ceramics are prized for their high hardness and wear resistance, making them ideal for components that require precision and longevity, such as mobile phone casings and camera lenses.
In the machinery sector, zirconia ceramics are utilized for their exceptional thermal stability and resistance to corrosive environments, which are crucial for components exposed to extreme conditions. This includes bearings, seals, and cutting tools that demand both strength and reliability.
The optical communications industry benefits from zirconia's transparency in the infrared spectrum, enabling its use in optical fibers and lenses that facilitate high-speed data transmission. Additionally, its chemical inertness ensures that these components remain unaffected by the materials they interact with, maintaining optical clarity and performance over time.
Within the chemical and medical industries, zirconia ceramics are employed for their biocompatibility and resistance to chemical attack. In chemical processing, they are used in valves and pumps that handle corrosive fluids, while in medical applications, they are incorporated into prosthetics and implants due to their non-toxic nature and ability to integrate seamlessly with human tissue.

The automobile and aviation sectors also make significant use of zirconia ceramics. In automobiles, they are found in engine components that require high thermal resistance and mechanical strength, such as turbochargers and exhaust systems. In aviation, zirconia ceramics are used in turbine blades and other critical parts that must withstand the extreme temperatures and pressures of flight, ensuring both safety and efficiency.
| Industry | Application Examples | Key Properties Utilized |
|---|---|---|
| 3C Electronics | Mobile phone casings, camera lenses | High hardness, wear resistance |
| Machinery | Bearings, seals, cutting tools | Thermal stability, corrosion resistance |
| Optical Communications | Optical fibers, lenses | Infrared transparency, chemical inertness |
| Chemical & Medical | Valves, pumps, prosthetics, implants | Biocompatibility, chemical resistance |
| Automobile & Aviation | Turbochargers, exhaust systems, turbine blades | Thermal resistance, mechanical strength |
These diverse applications underscore the versatility and robustness of zirconia ceramics, positioning them as a critical material in modern industrial and commercial sectors.
Future Development
The future development of zirconium oxide powder is poised to evolve towards achieving higher purity, ultrafine particle sizes, and enhanced stability, all while avoiding agglomeration and ensuring excellent uniformity. This progression is not merely a refinement of existing properties but a strategic advancement aimed at unlocking new functionalities and expanding into previously uncharted fields.
To illustrate, consider the potential applications in the biomedical sector. High-purity, ultrafine zirconia powders could be instrumental in the development of biocompatible implants with superior mechanical properties, such as dental implants and orthopedic prosthetics. The absence of agglomeration ensures that these materials can be processed into intricate shapes with minimal defects, thereby improving their overall performance and longevity.
| Development Aspect | Current State | Future Goal | Potential Applications |
|---|---|---|---|
| Purity | Moderately high | High purity | Biomedical implants, advanced electronics |
| Particle Size | Fine | Ultrafine | High-resolution coatings, precision machining |
| Agglomeration | Some agglomeration | No agglomeration | Uniform ceramic bodies, defect-free components |
| Uniformity | Good | Excellent | Consistent material properties, reliable performance |
| Stability | Stable | Highly stable | Long-term durability, resistance to environmental factors |
Moreover, the expansion of zirconia product manufacturing into new functions and fields could revolutionize industries ranging from electronics to environmental protection. For instance, zirconia ceramics could be engineered to serve as catalysts in environmentally friendly chemical processes, reducing the need for harmful chemicals and lowering energy consumption.
In summary, the future trajectory of zirconia oxide powder development is set to be a dynamic journey towards unparalleled purity, precision, and versatility, opening up a myriad of possibilities across diverse sectors.
Related Products
- Precision Machined Yttria Stabilized Zirconia Ceramic Plate for Engineering Advanced Fine Ceramics
- Custom-Made Alumina Zirconia Special-Shaped Ceramic Plates for Engineering Advanced Fine Ceramics Processing
- Precision Machined Yttrium Stabilized Zirconia Ceramic Rod for Engineering Advanced Fine Ceramics
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
Related Articles
- Unlocking the Power of Optical Quartz Plates: Applications and Benefits
- The Latest Advancements in Zirconia Sintering Furnaces for Dental Applications
- Top 5 Features of a High-Quality Zirconia Sintering Oven
- An In-depth Study of Isostatic Presses: Types, Applications, and Advantages
- Unveiling the Exceptional Properties and Applications of Optical Quartz Plates