Signs of a Bad Reference Electrode
IR Voltage Drop
In cyclic voltammetry (CV) tests, a substantial resistance within the reference electrode can lead to a voltage shift in the spectrogram, thereby distorting the interpretation of polarization behavior. This shift can result in misleading conclusions about the electrochemical processes occurring at the electrode surface. Similarly, in electrochemical impedance spectroscopy (EIS) tests, a high resistance can introduce a significant offset in the Rs value at the intersection with the X-axis. This offset can obscure the true impedance characteristics of the system, making it difficult to accurately diagnose and address the underlying issues.
To better understand the impact of resistance on these measurements, consider the following scenarios:
-
CV Tests: A high resistance in the reference electrode can cause a voltage shift that misrepresents the actual polarization curve. This shift can be particularly problematic when trying to identify peak currents or specific voltage ranges associated with certain reactions.
-
EIS Tests: In EIS, the resistance can lead to an offset in the Rs value, which is crucial for determining the system's impedance at different frequencies. This offset can skew the impedance spectrum, making it challenging to distinguish between capacitive and resistive components.
| Test Type | Impact of High Resistance | Corrective Measures |
|---|---|---|
| CV | Voltage shift in spectrogram | Ensure low resistance in reference electrode |
| EIS | Offset in Rs value | Use techniques to reduce impedance at high frequencies |
Addressing these issues requires careful calibration and monitoring of the reference electrode's resistance. Techniques such as parallel capacitor connections in EIS can help mitigate high-frequency artifacts, ensuring more accurate impedance measurements.
High-Frequency Artifacts
In Electrochemical Impedance Spectroscopy (EIS) tests, high-frequency artifacts can manifest as circular patterns in the impedance spectrum. These artifacts are often attributed to the reference electrode, which can introduce unwanted impedance at higher frequencies. The presence of these artifacts can obscure the true impedance characteristics of the system, leading to inaccurate data interpretation.
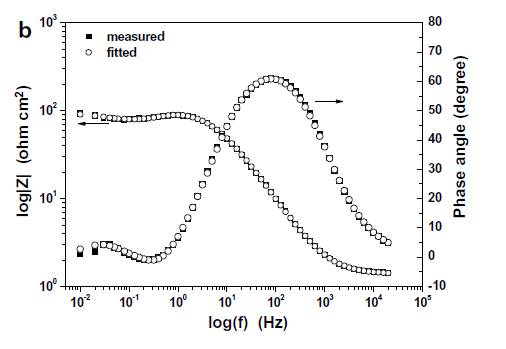
To mitigate this issue, one effective strategy is to reduce the impedance at high frequencies without compromising the low-frequency impedance. This can be achieved by incorporating a capacitor in parallel with the reference electrode. The capacitor acts as a low-impedance path at high frequencies, effectively bypassing the reference electrode and reducing the overall impedance.
| Frequency Range | Impedance Effect | Mitigation Strategy |
|---|---|---|
| High Frequency | Increased Impedance | Connect a capacitor in parallel |
| Low Frequency | Unaffected Impedance | No change needed |
By strategically placing a capacitor, the high-frequency artifacts can be significantly reduced, allowing for clearer and more accurate EIS spectra. This approach ensures that the reference electrode remains functional and reliable across a broad range of frequencies, enhancing the overall quality of the EIS data.
Identifying a Bad Reference Electrode
OCV Value Analysis
One method to identify a bad reference electrode is by measuring the Open Circuit Voltage (OCV) value between the reference electrode and a stable working electrode. This technique allows for the monitoring of the reference electrode's performance over time. Significant changes in the OCV value can serve as a clear indicator of potential issues with the reference electrode. Such changes might manifest as abrupt shifts or gradual drifts in the OCV readings, which could compromise the accuracy of subsequent measurements.
To perform this analysis, a stable working electrode is essential to provide a reliable baseline against which the reference electrode's OCV can be compared. A table summarizing typical OCV values and their corresponding conditions can be particularly useful for diagnosing issues:
| Condition | Typical OCV Value |
|---|---|
| Healthy Reference Electrode | Stable, no drift |
| Degraded Reference Electrode | Gradual drift |
| Faulty Reference Electrode | Abrupt shift |
By regularly monitoring the OCV values and comparing them against these benchmarks, it becomes easier to detect and address any anomalies that might arise. This proactive approach not only helps in maintaining the integrity of the measurement system but also ensures that any necessary corrective actions can be taken promptly.
EIS Test Analysis
Electrochemical Impedance Spectroscopy (EIS) is a critical tool for diagnosing the health of a reference electrode. When conducting an EIS test, the impedance of the reference electrode is meticulously measured across a range of frequencies. If the impedance of the reference electrode exceeds 1kΩ, it signals a potential issue that may require intervention.
This impedance threshold is not arbitrary; it is derived from the need to maintain accurate and reliable measurements. A reference electrode with impedance above 1kΩ can introduce significant errors in the EIS spectrum, manifesting as distortions or offsets in the data. These errors can obscure the true behavior of the system, leading to incorrect interpretations of the electrochemical processes at play.
For instance, in systems where the reference electrode is used to monitor the potential of a working electrode, high impedance can cause a voltage drop, known as the IR drop, which can skew the measurements. This is particularly problematic in applications where precise potential control is essential, such as in battery research or corrosion studies.
| Impedance Range | Potential Impact | Recommended Action |
|---|---|---|
| < 1kΩ | Minimal distortion | Continue monitoring |
| > 1kΩ | Significant errors | Adjust or replace |
In cases where the impedance exceeds the critical threshold, the reference electrode may need to be adjusted or replaced to restore accuracy. Adjustments could involve reconditioning the electrode through specific cleaning or re-plating processes. If these measures fail, replacing the reference electrode with a new one is often the most effective solution to ensure continued reliable measurements.
By regularly performing EIS tests and monitoring the impedance of reference electrodes, researchers and engineers can proactively manage potential issues, ensuring the integrity and accuracy of their electrochemical measurements.
c
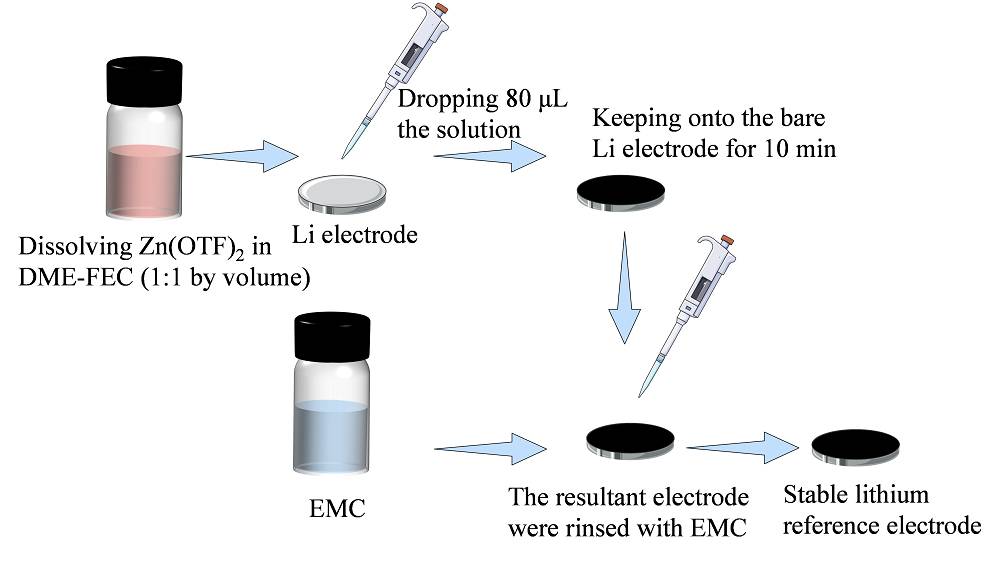
Lithium Metal Reference Electrode
When dealing with lithium metal reference electrodes that exhibit unstable open-circuit voltage (OCV) values, a strategic approach is often necessary to stabilize their performance. One effective method involves repeating the lithium plating process, which can help in restoring the electrode's reliability. This process typically involves carefully controlling the deposition of lithium onto the electrode surface, ensuring a uniform and stable layer is formed.
However, if the issue persists even after multiple attempts, and the electrode continues to show irregularities during electrochemical impedance spectroscopy (EIS) spectral tests, it may be indicative of deeper issues within the electrode's structure or material integrity. In such cases, creating a new reference electrode becomes a necessary step. This involves the meticulous fabrication of a new electrode, adhering to strict quality control measures to ensure it meets the required standards for stability and accuracy.
| Issue | Solution |
|---|---|
| Unstable OCV values | Repeat lithium plating process |
| Persistent EIS irregularities | Create a new reference electrode |
By addressing these issues proactively, the accuracy and reliability of the measurement system can be significantly enhanced, ensuring more precise and consistent data collection in electrochemical studies.
Redundancy in Reference Electrodes
Implementing redundancy in battery design by incorporating multiple reference electrodes serves as a robust strategy to mitigate the risks associated with a malfunctioning or unusable reference electrode. This approach not only enhances the reliability of the measurement system but also ensures consistent data accuracy, which is crucial for both research and practical applications.
In scenarios where a single reference electrode fails, having a backup immediately available prevents data loss and maintains the integrity of the experiments. This redundancy can be particularly beneficial in critical applications such as medical devices or aerospace systems, where the reliability of data is paramount.
Moreover, the use of multiple reference electrodes can facilitate more comprehensive diagnostic tests. For instance, comparing the readings from different electrodes can help identify subtle anomalies that might go unnoticed with a single electrode setup. This comparative analysis can provide deeper insights into the performance and health of the battery, aiding in more effective troubleshooting and maintenance.
| Benefit | Description |
|---|---|
| Enhanced Reliability | Ensures consistent data accuracy by having backup reference electrodes. |
| Prevent Data Loss | Immediate backup available in case of a failed reference electrode. |
| Comprehensive Diagnostics | Allows for comparative analysis, identifying subtle anomalies. |
| Critical Applications | Essential for high-stakes fields like medical devices and aerospace systems. |
By integrating redundancy in reference electrodes, the overall robustness and reliability of the battery system are significantly improved, making it a valuable strategy in the design and implementation of advanced measurement systems.
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
- Electrode Fixture for Electrochemical Experiments
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Flat Corrosion Electrolytic Electrochemical Cell
Related Articles
- Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
- Electrochemical Electrodes in Chemical Analysis
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
- A Comprehensive Guide to Reference Electrodes
- Electrolytes and Electrochemical Electrodes












