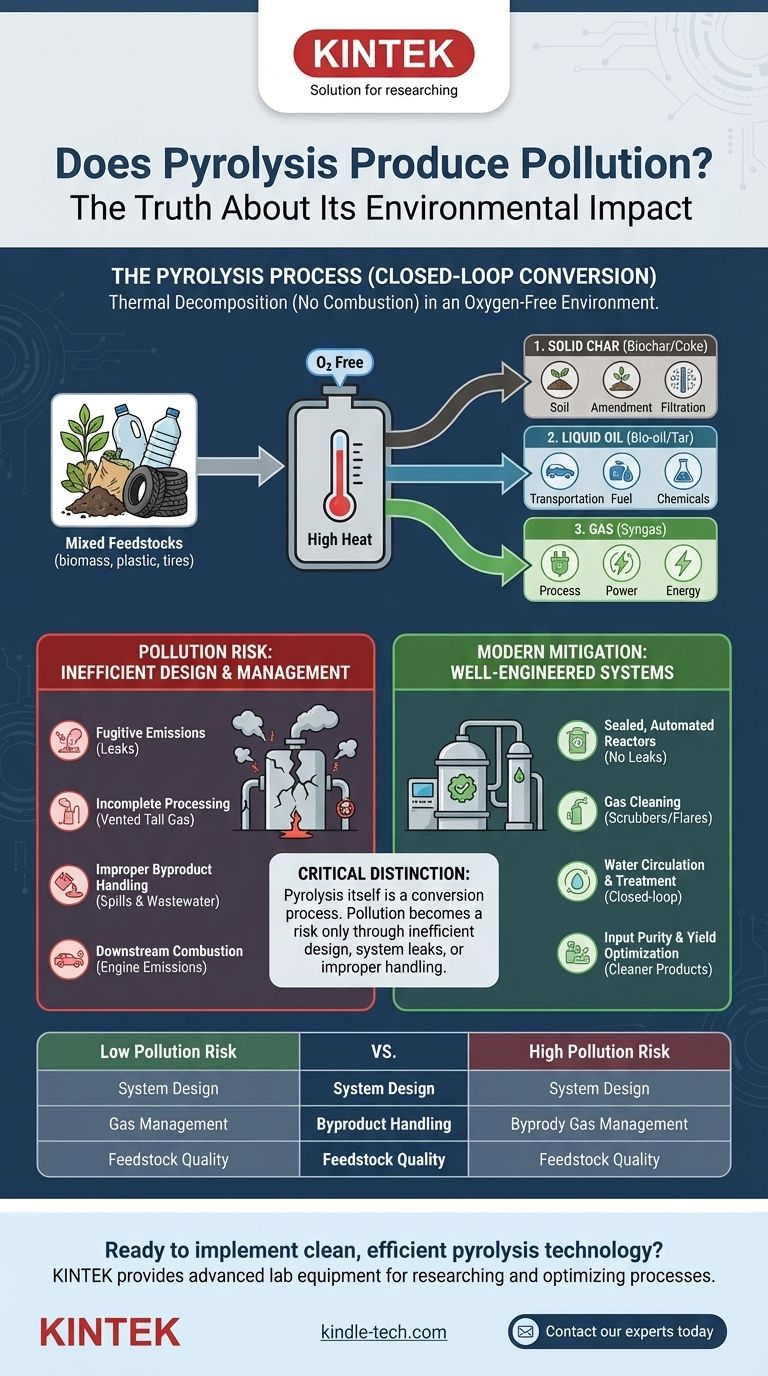
In principle, pyrolysis is a closed-loop process designed to minimize pollution, but its environmental impact depends entirely on the system's design and operational integrity. Unlike incineration, which burns material and releases flue gases, pyrolysis uses high heat in an oxygen-free environment to thermally decompose material. The primary outputs—a solid char, a liquid oil, and a combustible gas—are captured as valuable products. The potential for pollution arises not from the core process, but from how these outputs are managed and whether the system is properly sealed and maintained.
The critical distinction is that pyrolysis itself is not an act of pollution; it is a conversion process. Pollution becomes a risk only through inefficient design, system leaks, or the improper handling and combustion of its outputs. A modern, well-engineered plant is designed to capture and neutralize these potential emission points.

How Pyrolysis Works: A Contained Conversion Process
To understand the pollution potential, we must first understand the fundamental mechanism. Pyrolysis is not burning; it is a chemical breakdown driven by heat alone.
The Core Reaction
The process involves heating a feedstock, such as biomass, plastic, or tires, in a sealed reactor without oxygen. This prevents combustion and instead forces the complex organic molecules to break down into simpler, more stable components.
The Three Primary Outputs
This decomposition reliably sorts the material into three distinct streams:
- Solid (Biochar/Coke): A carbon-rich solid that can be used as a soil amendment, for filtration, or as an energy source.
- Liquid (Bio-oil/Tar): A dense liquid that can be refined into transportation fuels, used in boilers, or serve as a source for specialty chemicals.
- Gas (Syngas): A mixture of combustible gases, primarily carbon monoxide and hydrogen. Most modern pyrolysis plants use this gas to power the process itself, creating a self-sustaining energy loop.
Feedstock Determines the Output
The exact composition of these products depends heavily on the input material. Pyrolyzing wood yields biochar and bio-oil, while pyrolyzing methane primarily produces solid carbon and clean-burning hydrogen gas.
Identifying the Real Pollution Risks
A perfectly designed and operated pyrolysis plant would have near-zero unplanned emissions. However, in practice, risks emerge from system imperfections and byproduct management.
Fugitive Emissions
Any industrial plant with gases under pressure faces the risk of leaks. If the pyrolysis reactor or its associated piping is not perfectly sealed, volatile organic compounds (VOCs) or other gases can escape into the atmosphere.
Incomplete Processing (Tail Gas)
A pyrolysis plant may not be able to consume 100% of the syngas it produces. This excess gas, or "tail gas," must be handled properly. Simply venting it would release pollutants. Modern systems re-route this gas through a cleaning system or a flare to combust it safely.
Handling of Byproducts
The captured bio-oil and solid char can contain contaminants present in the original feedstock. If spilled or improperly stored, these can pollute soil and water. Likewise, water used for cooling or cleaning must be treated in a closed-loop system to prevent the release of contaminated wastewater.
Downstream Combustion
While the pyrolysis plant itself may be low-emission, the products it creates are often intended for fuel. Burning the bio-oil or syngas in an engine or turbine will produce its own emissions, such as NOx and SOx, which must be managed just like any other fuel.
Understanding the Trade-offs and Mitigation
The difference between a clean pyrolysis facility and a polluting one comes down to engineering, operation, and the quality of the input material.
The Importance of Modern Engineering
As the references highlight, modern waste pyrolysis plants are equipped with extensive pollution control systems. These include:
- Smoke and Tail Gas Cleaning: Scrubbers or filters that remove particulates and neutralize harmful compounds from any excess gas before it is released.
- Sealed, Automated Systems: Preventing fugitive emissions through high-integrity seals and automated feeding/discharging systems.
- Water Circulation Systems: Treating and reusing all process water to ensure no contaminated liquid is discharged.
Input Purity Matters
The process concentrates the elements from the feedstock. If you pyrolyze tires containing sulfur or plastics containing heavy metals, those elements will be concentrated in the oil and char. Using a cleaner, more homogenous feedstock results in cleaner, more valuable end products with lower environmental risk.
Process Conditions Dictate Yield
Operators can fine-tune the process to prioritize certain outputs. Lower temperatures (400–500 °C) favor the production of stable biochar, which is excellent for carbon sequestration. Higher temperatures (above 700 °C) maximize the yield of liquid and gaseous fuels, shifting the environmental focus to their eventual combustion.
Evaluating a Pyrolysis Project's Environmental Impact
To determine if a specific pyrolysis application is polluting, you must look beyond the core technology and analyze the entire operational plan.
- If your primary focus is waste management: Scrutinize the plant's engineering for robust, proven controls for tail gas, wastewater, and fugitive emissions.
- If your primary focus is producing clean fuel: Analyze the entire lifecycle, including the emissions profile of burning the resulting bio-oil or syngas.
- If your primary focus is carbon sequestration: Verify the stability and purity of the resulting biochar and the protocols for its safe application to soil.
Ultimately, pyrolysis is a powerful tool whose environmental performance is defined not by its theoretical potential, but by its real-world execution.
Summary Table:
| Factor | Low Pollution Risk | High Pollution Risk |
|---|---|---|
| System Design | Sealed, automated reactor with gas cleaning | Leaky, poorly sealed system with open vents |
| Gas Management | Syngas used for process heat; excess gas flared/cleaned | Tail gas vented directly to atmosphere |
| Byproduct Handling | Bio-oil and char stored properly; wastewater treated/recycled | Spills, improper storage, and contaminated water discharge |
| Feedstock Quality | Clean, homogenous materials (e.g., wood, biomass) | Contaminated waste (e.g., tires with sulfur, plastics with heavy metals) |
Ready to implement clean, efficient pyrolysis technology in your operations?
KINTEK specializes in advanced lab equipment and consumables for researching and optimizing pyrolysis processes. Whether you're developing new materials, analyzing biochar, or testing syngas composition, our precise and reliable tools help you minimize environmental impact and maximize product value.
Contact our experts today to find the right solutions for your laboratory's pyrolysis and thermal conversion needs.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- What materials are used for the tubes in tube furnaces? A Guide to Selecting the Right Tube for Your Process
- What are the advantages of a tube furnace? Achieve Superior Thermal Control and Purity
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?



















