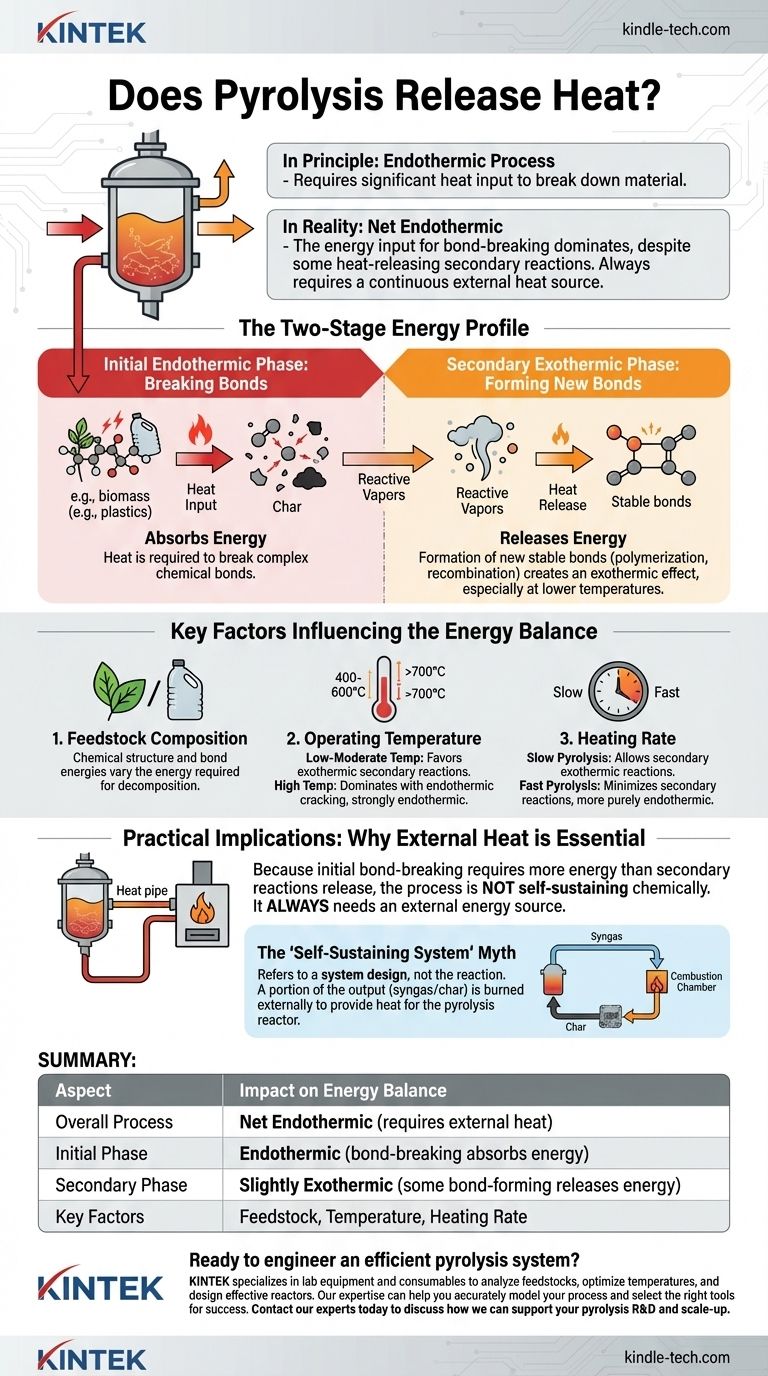
In principle, pyrolysis is an endothermic process, meaning it requires an input of heat to break down material. However, the complete energy balance is more complex. While the initial thermal decomposition of the feedstock absorbs energy, subsequent secondary reactions can release a small amount of heat, making the overall process less endothermic than it might seem at first glance.
While certain secondary reactions within pyrolysis can release heat (exothermic), the overall process is dominated by the energy required to break down the feedstock. Therefore, for all practical purposes, a pyrolysis system is considered net endothermic and always requires a continuous external heat source to operate.

The Two-Stage Energy Profile of Pyrolysis
To understand the energy flow, it's best to view pyrolysis as a process with two competing thermal stages: an initial energy-absorbing stage and a secondary energy-releasing stage.
The Initial Endothermic Phase: Breaking Bonds
Pyrolysis is, by definition, the thermal decomposition of organic material in the absence of oxygen. Breaking the complex and stable chemical bonds within materials like biomass (cellulose, lignin) or plastics requires a significant amount of energy.
This initial phase is always endothermic. It absorbs heat from the reactor environment to initiate and sustain the breakdown of large molecules into smaller, volatile compounds and solid char.
The Secondary Exothermic Phase: Forming New Bonds
Once the initial breakdown occurs, the resulting highly reactive vapors and radicals can undergo further reactions. These are known as secondary reactions.
Some of these reactions, such as polymerization and recombination, form new, more stable chemical bonds in the gas or solid (char) phase. The formation of more stable bonds releases energy, creating an exothermic effect. This effect is most notable at lower pyrolysis temperatures where these reactions have more time to occur.
Key Factors That Influence the Energy Balance
The exact balance between endothermic and exothermic reactions is not fixed. It depends heavily on the feedstock and operating conditions of the reactor.
Feedstock Composition
Different materials have different chemical structures and bond energies. The decomposition of the primary components of biomass—cellulose, hemicellulose, and lignin—is globally endothermic. However, the specific energy required varies for each.
Operating Temperature
Temperature is a critical factor.
- Low to Moderate Temperatures (400-600°C): In this range, there is a greater chance for exothermic secondary reactions (like char formation) to occur, which can slightly offset the initial energy input.
- High Temperatures (>700°C): At higher temperatures, the process is dominated by endothermic cracking reactions that break molecules down even further. This makes high-temperature pyrolysis strongly endothermic.
Heating Rate (Process Type)
The speed at which the material is heated determines which reactions are favored.
- Slow Pyrolysis: Longer residence times allow secondary exothermic reactions to proceed. This can slightly reduce the overall net energy required by the system.
- Fast Pyrolysis: This process aims to maximize liquid yield by rapidly heating the material and quickly removing the vapors. This minimizes secondary reactions, making the process more purely endothermic.
Understanding the Practical Implications
From an engineering and operational standpoint, the net endothermic nature of pyrolysis is the most important takeaway.
Why Pyrolysis Reactors Always Need External Heat
Because the initial energy required to break down the feedstock is greater than the energy released by secondary reactions, the process is not self-sustaining. A pyrolysis reactor always requires a constant and significant external energy source to maintain its operating temperature.
The Self-Sustaining System Myth
You may hear references to "self-sustaining" pyrolysis. This does not mean the chemical reaction itself provides the energy. It refers to a clever system design where a portion of the outputs—typically the non-condensable syngas or some of the char—is burned in an external chamber to provide the heat for the pyrolysis reactor.
The core pyrolysis reaction remains endothermic; the overall system is simply designed to power itself by consuming some of its own products.
How to Apply This to Your Goal
Your focus determines which aspect of the energy balance matters most.
- If your primary focus is designing an efficient reactor: You must engineer a robust external heating mechanism, as the process is fundamentally net endothermic. Your goal is to deliver heat as efficiently as possible.
- If your primary focus is evaluating the economics of a pyrolysis plant: Factor in the significant energy cost required to run the reactor, but also evaluate the potential to use the product gas or char to offset that energy input, improving the overall energy balance of the facility.
- If your primary focus is understanding the basic science: Remember that pyrolysis is a balance between bond-breaking (endothermic) and bond-forming (exothermic), with the former dominating the overall energy demand.
Understanding this fundamental energy balance is the first step toward engineering an effective and economically viable pyrolysis system.
Summary Table:
| Aspect | Impact on Energy Balance |
|---|---|
| Overall Process | Net Endothermic (requires external heat) |
| Initial Phase | Endothermic (bond-breaking absorbs energy) |
| Secondary Phase | Slightly Exothermic (some bond-forming releases energy) |
| Key Influencing Factors | Feedstock type, operating temperature, heating rate |
Ready to engineer an efficient pyrolysis system?
Understanding the energy balance is just the first step. KINTEK specializes in the lab equipment and consumables needed to analyze feedstocks, optimize temperatures, and design effective reactors. Our expertise can help you accurately model your process and select the right tools for success.
Contact our experts today to discuss how we can support your pyrolysis R&D and scale-up.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy



















