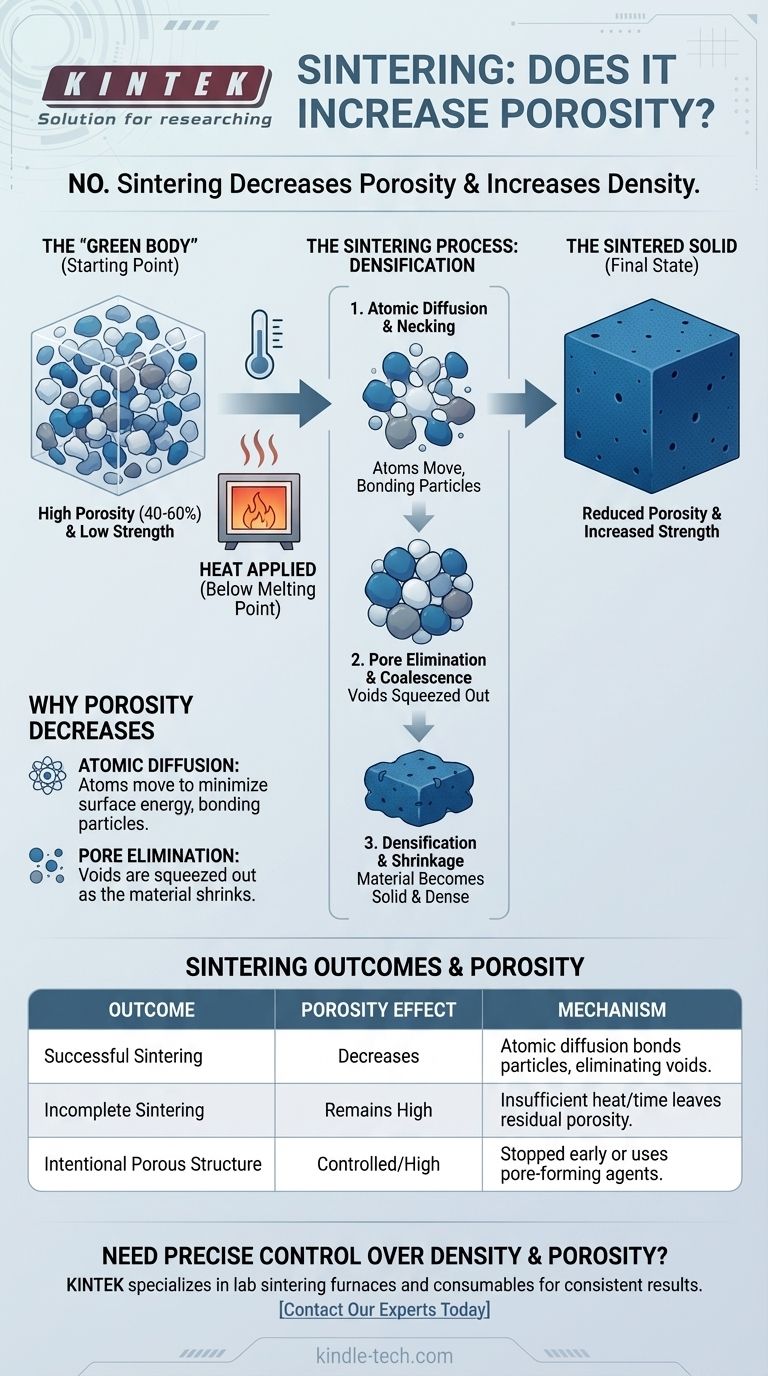
On the contrary, the fundamental purpose of the sintering process is to decrease porosity and increase the density of a material. It transforms a porous collection of individual particles, known as a green body, into a solid, coherent mass by applying heat below the material's melting point. This process causes the particles to fuse, systematically eliminating the voids between them.
Sintering is fundamentally a process of densification. It does not increase porosity; it reduces it by using thermal energy to drive atomic diffusion, which bonds particles together and closes the empty spaces that define a material's porosity.

The Goal of Sintering: From Powder to Solid
To understand why sintering reduces porosity, you must first visualize the starting material. The process begins not with a solid block, but with a loosely compacted powder or "green body."
The "Green Body": A High-Porosity Starting Point
A green body is the initial, unsintered component, typically formed by pressing a powder into a desired shape. This part is mechanically weak and characterized by a high degree of porosity—often between 40% and 60% of its total volume consists of empty space.
The Mechanism: Atomic Diffusion
When heated, atoms on the surfaces of adjacent particles become mobile. They begin to move, or diffuse, across the particle boundaries. This movement is the engine of sintering.
Instead of melting and flowing, the material transports itself on an atomic scale to minimize its surface energy. The lowest energy state is a single, dense solid, not a collection of individual particles with vast surface area.
Necking: The First Stage of Fusion
The first observable stage of sintering is called necking. At the points where particles touch, diffusion creates small bridges or "necks" of solid material. As these necks grow, they pull the centers of the particles closer together.
Pore Elimination and Densification
As the necks expand, the small, individual voids between particles coalesce and are gradually filled by the diffusing atoms. The empty spaces are effectively squeezed out of the structure.
This elimination of pores causes the entire component to shrink and become denser. The reduction in porosity is directly linked to an increase in density, strength, and other mechanical properties.
When Sintering Seems to Fail
While the goal is always to reduce porosity, certain conditions or phenomena can limit the effectiveness of sintering or, in very rare cases, create new voids. Understanding these is key to process control.
Incomplete Sintering
The most common reason for a sintered part to remain porous is simply incomplete sintering. If the temperature is too low or the time is too short, the diffusion process does not run to completion, leaving behind a network of residual porosity. This doesn't increase porosity from the initial state, but it fails to eliminate it.
Gas Entrapment
As sintering progresses, pores shrink. If a pore becomes isolated from the surface before it is fully eliminated, any gas trapped inside (like air or atmospheric gases) can become pressurized. This internal pressure can push back against the sintering forces, preventing the pore from closing completely.
The Kirkendall Effect (A Special Case)
In alloys made from different metals with vastly different diffusion rates, a phenomenon called the Kirkendall effect can occur. One type of atom may diffuse into another particle faster than atoms diffuse back. This imbalance can lead to the formation of new voids, but this is a specific metallurgical phenomenon, not a general outcome of sintering.
Understanding the Trade-offs
Controlling the final porosity requires balancing several key process parameters. Pushing for maximum density is not always the optimal strategy.
Temperature and Time
These are the primary levers. Higher temperatures and longer hold times promote more atomic diffusion, leading to lower porosity. However, there is a limit.
Particle Size and Distribution
Finer, more uniform powders provide a greater driving force for sintering and pack more efficiently. This leads to lower starting porosity and a more uniform final microstructure, making it easier to achieve high density.
The Risk of Grain Growth
Excessive time at high temperatures can lead to oversintering. While this may eliminate porosity, it also causes the microscopic grains of the material to grow excessively large. Large grains can severely degrade mechanical properties like toughness and fatigue resistance, making the part brittle.
Achieving Your Desired Porosity
The "right" amount of porosity depends entirely on the application. Mastering sintering means learning to control it to achieve your specific goal.
- If your primary focus is maximum density and strength: Use fine, uniform powders and carefully optimize the temperature and time to close pores without causing excessive grain growth.
- If your primary focus is creating a porous structure (e.g., for filters or biomedical implants): Use larger, irregular particles, intentionally stop the sintering process early, or mix in a "pore-forming agent" that burns out during heating, leaving a deliberate network of open pores.
- If you are troubleshooting unexpected porosity: First, verify your sintering temperature and atmosphere are correct. Then, analyze your raw material—inconsistent particle size is a common culprit for non-uniform densification.
Ultimately, mastering sintering is about controlling atomic transport to achieve a precise, engineered microstructure.
Summary Table:
| Sintering Outcome | Effect on Porosity | Key Mechanism |
|---|---|---|
| Successful Sintering | Decreases | Atomic diffusion bonds particles, eliminating voids. |
| Incomplete Sintering | Remains High | Insufficient heat/time leaves residual porosity. |
| Intentional Porous Structure | Controlled/High | Process is stopped early or uses pore-forming agents. |
Need precise control over your material's density and porosity?
The sintering process is critical for achieving the mechanical properties your application demands. Whether your goal is maximum strength or a specific porous structure, KINTEK's expertise in lab sintering furnaces and consumables can help you optimize your process.
We specialize in providing reliable equipment and expert support for laboratories focused on material science and development. Let us help you achieve consistent, high-quality results.
Contact our experts today to discuss your sintering requirements!
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of muffle furnace in chemistry laboratory? Achieve Precise High-Temperature Material Processing
- What is the difference between hot air oven and muffle furnace? Choose the Right Tool for Your Lab's Thermal Needs
- What are the 3 official methods in determining ash and water content? A Guide to Proximate Analysis
- What is the main function of the muffle furnace? Achieve Pure, High-Temperature Heating Without Contamination
- What is the purpose of a muffle furnace in a lab? Achieve Pure, High-Temperature Heat for Your Materials



















